Molecular mechanisms and emerging therapeutics in pulmonary fibrosis: A recent update
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Pulmonary fibrosis (PF) is a chronic, progressive, and fatal lung disorder characterized by injury to alveolar epithelial cells (AECs), the formation of activated fibroblast/myofibroblast foci, and an excessive buildup of extracellular matrix (ECM). Current treatment strategies include the administration of two main drugs, viz., pirfenidone and nintedanib. However, there is no cure for PF; thus, there is a dire need to understand the pathophysiology of PF better and identify potential novel targets for PF. This review aims to provide a recent update on the signaling pathways contributing to the intricacies of cell signaling mechanisms driving fibrogenesis, including TGF-β, Hippo-YAP, NF-κB, inflammation, and Wnt/β-catenin. Additionally, the discussion covers emerging therapeutic strategies directed against such signaling networks, focusing on some of the most promising molecular strategies to halt or reverse fibrotic processes. Additionally, we discuss preclinical studies revealing the in vitro efficacy and safety, and the current clinical status of new therapeutic agents in PF. The synergy of preclinical and clinical studies' findings will allow this review to critically evaluate current therapeutic directions and emerging trends in PF management, shaping a future course of research and clinical application.
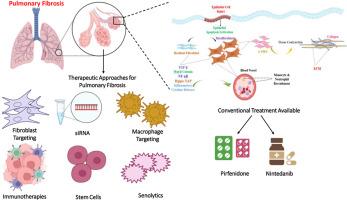
肺纤维化的分子机制和新兴治疗方法:最新进展。
肺纤维化(PF)是一种慢性、进行性和致死性肺部疾病,其特征是肺泡上皮细胞(AECs)损伤、活化成纤维细胞/肌成纤维细胞灶的形成以及细胞外基质(ECM)的过度积累。目前的治疗策略包括使用两种主要药物,即吡非尼酮和尼达尼布。然而,目前还没有治愈PF的方法;因此,迫切需要更好地了解PF的病理生理,并确定PF的潜在新靶点。本文旨在提供最近的信号通路,包括TGF-β、希波- yap、NF-κB、炎症和Wnt/β-catenin,这些信号通路有助于复杂的细胞信号机制驱动纤维化。此外,讨论涵盖了针对这些信号网络的新兴治疗策略,重点是一些最有希望阻止或逆转纤维化过程的分子策略。此外,我们还讨论了揭示体外疗效和安全性的临床前研究,以及PF新治疗药物的临床现状。临床前和临床研究结果的协同作用将使本综述能够批判性地评估PF管理的当前治疗方向和新趋势,从而形成未来的研究和临床应用过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


