Key roles of GAPDH, Hsp90, and NO in heme trafficking
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Intracellular trafficking of mitochondrial heme to create functional heme proteins presents a fundamental challenge in animal cells. This article provides some background on heme allocation, discusses some of the concepts, and then reviews research from the last two decades that has uncovered unexpected and important roles for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), heat shock protein 90 (Hsp90), and nitric oxide (NO) in enabling and regulating cell heme allocations to hemeproteins that mature and function outside of the mitochondria. A model for how hemeprotein heme contents and functions in cells can be regulated through the coordinate participation of GAPDH, Hsp90, and NO is presented.
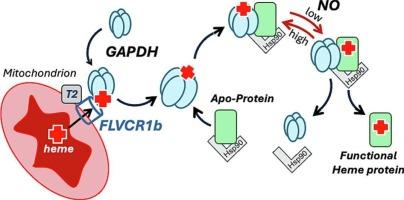
GAPDH、Hsp90和NO在血红素运输中的关键作用。
线粒体血红素在细胞内运输以产生功能性血红素蛋白是动物细胞的一个基本挑战。本文介绍了血红素分配的一些背景,讨论了一些概念,然后回顾了过去二十年来的研究,这些研究发现了甘油醛3-磷酸脱氢酶(GAPDH)、热休克蛋白90 (Hsp90)和一氧化氮(NO)在激活和调节细胞血红素分配到线粒体外成熟和功能的血红素蛋白中的意想不到的重要作用。提出了细胞中血红蛋白血红素含量和功能如何通过GAPDH、Hsp90和NO的协调参与来调节的模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


