Extrachromosomal DNA associates with nuclear condensates and reorganizes chromatin structures to enhance oncogenic transcription
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Extrachromosomal, circular DNA (ecDNA) is a prevalent oncogenic alteration in cancer genomes, often associated with aggressive tumor behavior and poor patient outcome. While previous studies proposed a chromatin-based mobile enhancer model for ecDNA-driven oncogenesis, its precise mechanism and impact remains unclear across diverse cancer types. Our study, utilizing advanced multi-omics profiling, epigenetic editing, and imaging approaches in three cancer models, reveals that ecDNA hubs are an integrated part of nuclear condensates and exhibit cancer-type specific chromatin connectivity. Epigenetic silencing of the ecDNA-specific regulatory modules or chemically disrupting nuclear condensates breaks down ecDNA hubs, displaces MED1 co-activator binding, inhibits oncogenic transcription, and promotes cell death. These findings substantiate the trans-activator function of ecDNA and underscore a structural mechanism driving oncogenesis. This refined understanding expands our views of oncogene regulation and opens potential avenues for alternative therapeutic strategies in cancer treatment.
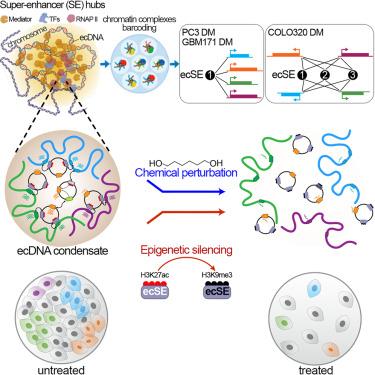
染色体外DNA与核凝聚体结合并重组染色质结构以增强致癌转录
染色体外环状DNA (ecDNA)是癌症基因组中普遍存在的致癌改变,通常与侵袭性肿瘤行为和不良患者预后相关。虽然先前的研究提出了一种基于染色质的移动增强子模型,用于ecdna驱动的肿瘤发生,但其在不同癌症类型中的确切机制和影响尚不清楚。我们的研究利用先进的多组学分析、表观遗传编辑和三种癌症模型的成像方法,揭示了ecDNA枢纽是核凝聚物的一个组成部分,并表现出癌症类型特异性的染色质连接。表观遗传沉默的ecDNA特异性调控模块或化学破坏核凝聚物破坏ecDNA枢纽,取代MED1共激活子结合,抑制致癌转录,并促进细胞死亡。这些发现证实了ecDNA的反式激活子功能,并强调了驱动肿瘤发生的结构机制。这种完善的理解扩展了我们对癌基因调控的看法,并为癌症治疗的替代治疗策略开辟了潜在的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


