Restraint of cancer cell plasticity by spatial homotypic clustering
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Tumor heterogeneity fueled by plasticity of cancer cells is a key to therapy failure. Here, we define the role of proximal communications of malignant cells in glioblastoma plasticity. We find that tumor cell state coherence is maximal in cells organized in homotypic clusters with defined relationships with non-malignant cells, whereas randomly dispersed cells downregulate the original state, acquire alternative phenotypes and exhibit changes in the microenvironment. We demonstrate the intrinsic propensity of glioblastoma cells to develop into clustered and dispersed spatial patterns in orthotopic mouse models and experimentally validate the cell state-specific mechanisms of cell-cell adhesion that prevent phenotype deviation with pharmacologic perturbations in patients-derived glioblastoma models. We establish the generality of “homotypic clustered cell identity” in circulating clustered and single breast cancer cells and show that the glioblastoma glycolytic-plurimetabolic dispersed cellular state uniquely confers shorter survival, thus assigning clinical significance to the spatial patterning of cancer cells in human tumors.
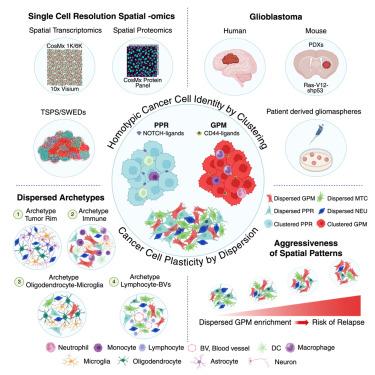
空间同型聚类对癌细胞可塑性的抑制
肿瘤细胞可塑性引发的肿瘤异质性是治疗失败的关键。在这里,我们定义了恶性细胞近端通讯在胶质母细胞瘤可塑性中的作用。我们发现肿瘤细胞状态一致性在与非恶性细胞有明确关系的同型细胞群中是最大的,而随机分散的细胞下调原始状态,获得不同的表型,并在微环境中表现出变化。我们在原位小鼠模型中证明了胶质母细胞瘤细胞发展成集群和分散空间模式的内在倾向,并通过实验验证了细胞状态特异性的细胞粘附机制,该机制可以防止患者源性胶质母细胞瘤模型中药物干扰引起的表型偏差。我们在循环的群集和单个乳腺癌细胞中建立了“同型群集细胞身份”的普遍性,并表明胶质母细胞瘤糖酵解-多代谢分散细胞状态独特地赋予较短的生存期,从而为人类肿瘤中癌细胞的空间模式赋予临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


