A novel indel-based molecular sex identification system for oplegnathus fasciatus: Insights from ndc80 gene polymorphisms and aquaculture applications
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-09-11
DOI:10.1016/j.cbd.2025.101632
引用次数: 0
Abstract
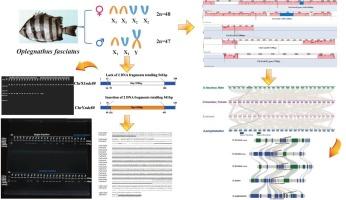
Oplegnathus fasciatus is a commercially important marine fish species, valued both in wild fisheries and aquaculture. It possesses a multivalent sex-determination system (X1X1X2X2/X1X2Y) and exhibits marked sexual growth dimorphism, with males demonstrating significantly faster growth rates. Sex-specific molecular markers are instrumental in advancing selective breeding strategies. In this study, whole-genome screening of O. fasciatus revealed a male-specific structural variant within an intronic region of ndc80: two insertions of 3 bp and 538 bp (totaling 541 bp), which were consistently absent in females. To facilitate practical application, we developed a PCR-based assay using a single primer pair that amplifies a conserved region flanking the insertion site. This assay reproducibly generates distinct banding patterns: males yield two fragments (208 bp and 749 bp), consistent with the 541 bp male-specific insertion, whereas females yield only the 208 bp amplicon. This method enables efficient, high-throughput sex identification in O. fasciatus without the need for sequencing. Using standard agarose gel electrophoresis, the assay reliably distinguishes sexes through clearly divergent banding patterns. Beyond applications in selective breeding, these sex-specific markers provide critical molecular insights into the sex determination mechanisms and the genetic basis of sexual dimorphism in O. fasciatus.
一种新的基于indle的筋膜鱼分子性别鉴定系统:来自ndc80基因多态性的见解及其在水产养殖中的应用
筋膜鱼是一种重要的商业海洋鱼类,在野生渔业和水产养殖中都很有价值。它具有多价性别决定系统(X1X1X2X2/X1X2Y),并表现出明显的性生长二态性,雄性的生长速度明显更快。性别特异性分子标记有助于推进选择性育种策略。在本研究中,对筋膜鄂鱼进行全基因组筛选,发现ndc80内含子区域存在男性特异性结构变异:两个3bp和538bp(总计541bp)的插入,而这在女性中一直不存在。为了便于实际应用,我们开发了一种基于pcr的检测方法,使用单个引物对扩增插入位点两侧的保守区域。该分析可重复地产生不同的条带模式:雄性产生两个片段(208bp和749bp),与541bp的雄性特异性插入一致,而雌性只产生208bp的扩增子。这种方法可以在不需要测序的情况下,高效、高通量地鉴定筋膜棘鱼的性别。使用标准琼脂糖凝胶电泳,该试验通过明显不同的条带模式可靠地区分性别。除了在选择性育种中的应用外,这些性别特异性标记还为筋膜鱼性别决定机制和性别二态性的遗传基础提供了重要的分子见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


