Autophagy and mitophagy in circulating immune cells of women with polycystic ovary syndrome: A cardiovascular perspective
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder associated with systemic inflammation, oxidative stress, and increased cardiometabolic risk. While impaired autophagy and mitophagy have been implicated in tissue-specific dysfunction, their role in circulating immune cells and relation to early vascular alterations remain unclear.
Methods
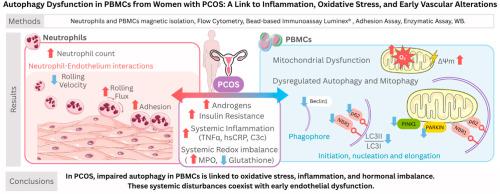
In this cross-sectional study, 91 women (48 controls, 43 with PCOS) underwent anthropometric and biochemical assessment. Systemic markers included myeloperoxidase (MPO), glutathione, TNFα, and sP-selectin were determined in serum. Superoxide production (dHE), mitochondrial membrane potential (TMRM), and protein levels of BECLIN1, LC3II/I, P62, NBR1, and PINK1 were assessed in peripheral blood mononuclear cells (PBMCs). Neutrophil-endothelial cell interactions were analysed as subclinical markers of endothelial dysfunction.
Results
PCOS patients presented hormonal, lipid, and glucose abnormalities, accompanied by redox imbalance, elevated MPO and superoxide levels, reduced glutathione, and increased mitochondrial membrane potential. Autophagy and mitophagy pathways were significantly impaired in PBMCs, and the expression of all assessed markers was reduced. These alterations were associated with androgen excess, oxidative stress, and inflammation. Elevated sP-selectin and enhanced neutrophil-endothelial interactions indicated early endothelial dysfunction in PCOS women.
Conclusion
Our findings reveal that women with PCOS display autophagy and mitophagy impairment in immune cells, processes that are linked to oxidative and inflammatory stress and accompanied by endothelial alterations, highlighting a potential mechanistic pathway contributing to their cardiometabolic risk.
多囊卵巢综合征妇女循环免疫细胞的自噬和有丝自噬:心血管角度
简介:多囊卵巢综合征(PCOS)是一种复杂的内分泌紊乱,与全身炎症、氧化应激和心脏代谢风险增加有关。虽然自噬和有丝自噬受损与组织特异性功能障碍有关,但它们在循环免疫细胞中的作用以及与早期血管改变的关系尚不清楚。方法:在这项横断面研究中,91名妇女(48名对照组,43名多囊卵巢综合征患者)进行了人体测量和生化评估。血清系统标志物包括髓过氧化物酶(MPO)、谷胱甘肽、TNFα和sp -选择素。检测外周血单个核细胞(PBMCs)的超氧化物生成(dHE)、线粒体膜电位(TMRM)以及BECLIN1、LC3II/I、P62、NBR1和PINK1蛋白水平。中性粒细胞-内皮细胞相互作用作为内皮功能障碍的亚临床标志物进行分析。结果:PCOS患者表现为激素、脂质、葡萄糖异常,伴氧化还原失衡,MPO、dHE水平升高,谷胱甘肽降低,线粒体膜电位升高。自噬和有丝自噬途径在PBMCs中明显受损,所有评估标志物的表达均降低。这些改变与雄激素过量、氧化应激和炎症有关。sp -选择素升高和中性粒细胞-内皮相互作用增强提示PCOS妇女早期内皮功能障碍。结论:我们的研究结果显示,患有多囊卵巢综合征的女性在免疫细胞中表现出自噬和有丝分裂损伤,这一过程与氧化和炎症应激有关,并伴有内皮改变,这一潜在的机制途径有助于增加其心脏代谢风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


