Activation of the Nrf2/HO-1/GPX4 pathway by cGAMP Mitigates oxidative stress and ferroptosis in ischaemic stroke
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Ischemic stroke(IS), a leading cause of neurological disability worldwide, involves intricate crosstalk between oxidative stress and regulated cell death pathways. In this study, we demonstrate that the immunomodulatory metabolite 2′3′-cyclic GMP-AMP (cGAMP) exerts neuroprotective effects using a mouse model of transient focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO), significantly decreased brain lesion size with concurrent amelioration of neurobehavioral outcomes through mechanisms distinct from canonical cGAS-STING signaling. cGAMP-dependent activation of the Nrf2/HO-1/GPX4 pathway was identified by multimodal interrogation as the primary mechanism curbing mitochondrial oxidative stress and lipid peroxidation, accompanied by ultrastructural preservation of mitochondrial cristae integrity and a reduction in ferroptotic markers. Pharmacological inhibition of Nrf2 using ML-385 or GPX4 with ML-210 completely abrogated these protective effects, thereby confirming pathway specificity. These findings establish cGAMP as a novel dual modulator of redox homeostasis and ferroptosis in ischemic stroke pathophysiology.
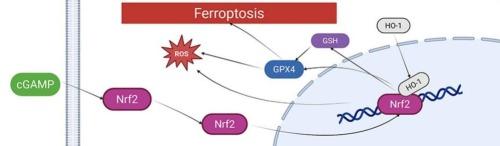
cGAMP激活Nrf2/HO-1/GPX4通路减轻缺血性脑卒中的氧化应激和铁凋亡。
缺血性中风(IS)是世界范围内神经功能障碍的主要原因,涉及氧化应激和调节细胞死亡途径之间复杂的串扰。在这项研究中,我们证明了免疫调节代谢物2'3'-环GMP-AMP (cGAMP)在大脑中动脉闭塞(MCAO)引起的短暂局灶性脑缺血小鼠模型中发挥神经保护作用,通过不同于典型cGAS-STING信号传导的机制,显著减少脑损伤大小,同时改善神经行为结果。通过多模式调查发现,Nrf2/HO-1/GPX4通路的cgamp依赖性激活是抑制线粒体氧化应激和脂质过氧化的主要机制,同时伴有线粒体嵴完整性的超微结构保存和铁致凋亡标志物的减少。ML-385或GPX4与ML-210对Nrf2的药理学抑制完全消除了这些保护作用,从而证实了通路的特异性。这些发现表明cGAMP在缺血性卒中病理生理中是氧化还原稳态和铁凋亡的一种新的双重调节剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


