Lac-Phe induces hypophagia by inhibiting AgRP neurons in mice
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
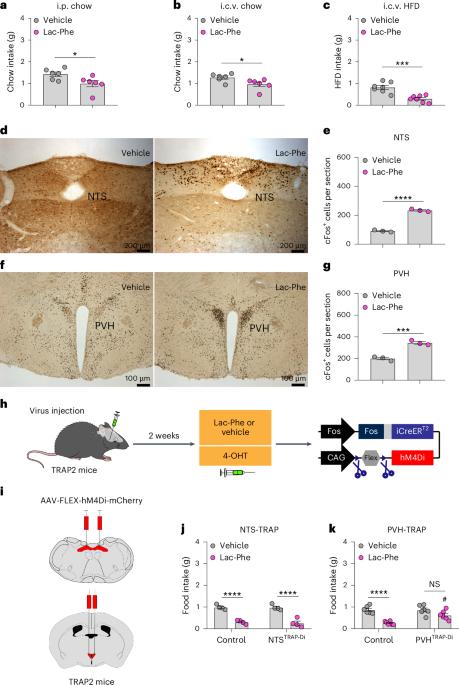
N-Lactoyl-phenylalanine (Lac-Phe) is a lactate-derived circulating metabolite that reduces feeding and obesity, but the molecular mechanisms that underlie the metabolic benefits of Lac-Phe remain unknown. Here we show that Lac-Phe directly inhibits hypothalamic neurons that express Agouti-related protein (AgRP), resulting in an indirect activation of anorexigenic neurons in the paraventricular nucleus of the hypothalamus (PVH). Both AgRP inhibition and PVH activation are required to mediate Lac-Phe-induced hypophagia. Lac-Phe-mediated inhibition of AgRP neurons occurs through activation of the ATP-sensitive potassium (KATP) channel, whereas inhibition of the KATP channel blunts the effects of Lac-Phe to suppress feeding. Together, these results reveal the molecular and neurobiological mechanisms by which Lac-Phe mediates metabolic improvements and suggest this exercise-induced metabolite might have therapeutic benefits in various human diseases. This study reveals neuronal targets of Lac-Phe in the hypothalamus that mediate its suppression of food intake.
Lac-Phe通过抑制AgRP神经元诱导小鼠吞咽
n -乳酸基苯丙氨酸(Lac-Phe)是一种乳酸衍生的循环代谢物,可减少摄食和肥胖,但Lac-Phe代谢益处的分子机制尚不清楚。本研究表明,Lac-Phe直接抑制表达agouti相关蛋白(AgRP)的下丘脑神经元,导致下丘脑室旁核(PVH)厌氧性神经元的间接激活。AgRP抑制和PVH激活都需要介导lac - phe诱导的吞咽。Lac-Phe介导的AgRP神经元的抑制是通过激活atp敏感钾(KATP)通道发生的,而KATP通道的抑制则减弱了Lac-Phe抑制进食的作用。总之,这些结果揭示了Lac-Phe介导代谢改善的分子和神经生物学机制,并表明这种运动诱导的代谢物可能对各种人类疾病有治疗作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


