Binding of Sunset Yellow, a food coloring, to human carbonic anhydrase II: Structural and functional insights from a multi-spectroscopic study
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The interaction of sunset yellow (SY), a widely used food coloring, with human carbonic anhydrase II (hCA II) was investigated using multiple spectroscopic techniques, including fluorescence, UV–Vis, and circular dichroism. The results showed that SY binds to hCA II, causing measurable changes in its enzymatic activity and structural properties. Fluorescence analyses indicated a quenching mechanism, while circular dichroism revealed alterations in secondary and tertiary structures. Moreover, SY binding affected the protein surface hydrophobicity index and its thermodynamic stability as well. Overall, these findings highlight that SY can modulate the structure and function of hCA II, providing insights with potential relevance to toxicology and enzymology.
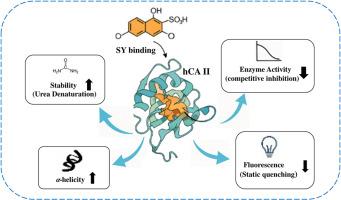
夕阳黄,一种食用色素,与人类碳酸酐酶II的结合:多光谱研究的结构和功能见解。
采用荧光、紫外-可见和圆二色等多种光谱技术研究了落日黄(SY)与人类碳酸酐酶II (hCA II)的相互作用。结果表明,SY与hCA II结合,导致其酶活性和结构性质发生可测量的变化。荧光分析表明了猝灭机制,而圆二色性显示了二级和三级结构的改变。此外,SY结合还影响了蛋白质的表面疏水性指数及其热力学稳定性。总的来说,这些发现强调了SY可以调节hCA II的结构和功能,提供了与毒理学和酶学潜在相关的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


