Mutational effects of the asparagine198 and glutamate223 residues on the human norepinephrine transporter on basal and HIV-1 Tat protein-induced inhibition of dopamine transport
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
HIV-1 transactivator of transcription (Tat) protein induces dopaminergic dysregulation, which plays a central role in HIV-1-associated neurocognitive disorders. Computational modeling predicts that asparagine 198 and glutamate 223 of the human norepinephrine transporter (hNET) are key residues involved in Tat binding. This study investigated the effects of N198A and E223A mutations on basal and Tat-induced inhibition of dopamine (DA) uptake via hNET in CHO cells expressing WT hNET or its mutants. Compared to WT hNET, E223A mutation increased the affinity for nisoxetine and cocaine in inhibiting [3H]DA uptake. However, N198A and E223A decreased the affinity for cocaine inhibiting [3H]WIN35,428 binding, without altering the [3H]WIN35,428 binding under control. Kinetic analysis of [3H]DA uptake revealed that N198A and E223A did not alter the affinity for DA uptake but reduced the maximal velocity compared to WT hNET. An optimization study using recombinant Tat1-86 at 0.25–140 nM revealed a Ki of 3.4 nM for inhibiting hNET-mediated DA uptake, with inhibition plateauing at above 8.75 nM. Treatment with 140 nM recombinant Tat1-86 resulted in a 34 % reduction in [3H]DA uptake in WT hNET, which was attenuated in the N198A mutant but remained unchanged in E223A. However, the inhibition of [3H]DA uptake by 8.75 nM rTat1-86 in WT hNET was attenuated in N198A and E223A. Moreover, N198A and E223A altered transporter conformational dynamics, as evidenced by changing the efflux of [3H]DA and [3H]MPP+. Collectively, these findings support the role of asparagine198 and glutamate223 as essential recognition residues in Tat-induced inhibition of DA uptake through hNET.
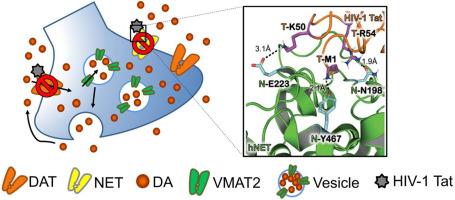
人去甲肾上腺素转运蛋白上的天冬酰胺198和谷氨酸223残基对基础和HIV-1 Tat蛋白诱导的多巴胺转运抑制的突变效应
HIV-1转录反激活因子(Tat)蛋白诱导多巴胺能失调,这在HIV-1相关的神经认知障碍中起着核心作用。计算模型预测,人去甲肾上腺素转运体(hNET)的天冬酰胺198和谷氨酸223是参与Tat结合的关键残基。本研究探讨了N198A和E223A突变对表达WT hNET或其突变体的CHO细胞通过hNET摄取多巴胺(DA)的基础抑制和tat诱导的抑制作用。与WT hNET相比,E223A突变增加了对尼索西汀和可卡因的亲和力,抑制[3H]DA摄取。然而,N198A和E223A降低了可卡因抑制[3H]WIN35,428结合的亲和力,而不改变控制下的[3H]WIN35,428结合。对[3H]DA摄取的动力学分析表明,与WT hNET相比,N198A和E223A没有改变对DA摄取的亲和力,但降低了最大速度。利用重组Tat1-86在0.25 ~ 140 nM进行优化研究,发现Ki为3.4 nM可抑制hnet介导的DA摄取,抑制在8.75 nM以上达到平台期。用140 nM重组Tat1-86处理后,WT hNET的[3H]DA摄取减少了34%,在N198A突变体中减少,但在E223A中保持不变。然而,8.75 nM rTat1-86对WT hNET中[3H]DA摄取的抑制作用在N198A和E223A中减弱。此外,N198A和E223A通过改变[3H]DA和[3H]MPP+的流出来改变转运体构象动力学。总的来说,这些发现支持了天冬酰胺198和谷氨酸223作为识别残基在tat诱导的通过hNET抑制DA摄取中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


