Aloe-emodin mitigates cisplatin-induced acute kidney injury by Nrf2-mediated ferroptosis regulation
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Acute kidney injury (AKI) is a common clinical critical illness, with currently limited treatment options available. Cisplatin (CDDP), a first-line drug for cancer chemotherapy, a significant contributor to nephrotoxicity, which severely restricts its clinical application. Therefore, there is an urgent need for pharmacological interventions to alleviate cisplatin (CDDP)-induced acute kidney injury (CI-AKI). Aloe-emodin (AE), as a novel ferroptosis inhibitor, shows great potential in mitigating cisplatin-induced acute kidney injury. This study aims to explore the effect and mechanism of aloe-emodin on cisplatin-induced acute kidney injury. By establishing in vitro and in vivo models, we evaluated indicators related to renal function, oxidative stress, ferroptosis, and the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway using techniques such as biochemical analysis, immunofluorescence, and Western blotting. The results indicated that AE significantly improves renal function indices, reduces levels of oxidative stress products, inhibits alterations in ferroptosis marker molecules, and alleviates renal pathological damage. Mechanistically, AE activates the Nrf2 pathway and upregulates the expression of its downstream antioxidant and anti-ferroptosis genes. Inhibition of the Nrf2 pathway significantly diminishes the protective effects of AE. This study suggests that AE mitigates CI-AKI by activating the Nrf2 pathway and inhibiting ferroptosis, thus providing new insights for clinical prevention and treatment.
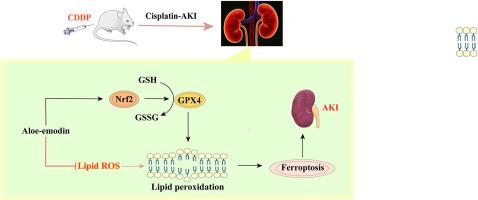
芦荟大黄素通过nrf2介导的铁下垂调节减轻顺铂诱导的急性肾损伤。
急性肾损伤(AKI)是一种常见的临床危重疾病,目前治疗方案有限。顺铂(CDDP)作为癌症化疗的一线药物,具有严重的肾毒性,严重制约了其临床应用。因此,迫切需要药物干预来减轻顺铂(CDDP)诱导的急性肾损伤(CI-AKI)。芦荟大黄素(AE)作为一种新型的铁下垂抑制剂,在减轻顺铂所致急性肾损伤方面显示出巨大的潜力。本研究旨在探讨芦荟大黄素对顺铂所致急性肾损伤的作用及机制。通过建立体外和体内模型,我们利用生化分析、免疫荧光和Western blotting等技术评估了肾功能、氧化应激、铁凋亡和核因子红细胞2相关因子2 (Nrf2)途径的相关指标。结果表明,AE能显著改善肾功能指标,降低氧化应激产物水平,抑制铁下垂标志物分子改变,减轻肾脏病理损害。在机制上,AE激活Nrf2通路,上调其下游抗氧化和抗铁下垂基因的表达。抑制Nrf2通路可显著降低AE的保护作用。本研究提示AE通过激活Nrf2通路,抑制铁下垂减轻CI-AKI,为临床预防和治疗提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


