Licochalcone B alleviates exercise-induced muscle fatigue through its antioxidant activity in experimental mice
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Muscle fatigue is a major constraint on physical performance and is driven by multiple factors such as excessive oxidative stress and ATP depletion. This study investigated the protective effects of licochalcone B (LicoB), a phenolic chalcone compound isolated from Glycyrrhiza glabra Linné, on oxidative stress-induced damage in C2C12 cells. Additionally, its anti-fatigue potential was evaluated in mice subjected to exhaustive swimming. In C2C12 myotubes, LicoB significantly reduced H2O2-induced oxidative damage and preserved myotube integrity by scavenging free radicals. Transcriptomic analysis of C2C12 cells showed that LicoB reversed stress-induced dysregulation of antioxidant and myogenic genes, indicating protective transcriptional reprogramming. An in vivo mouse model employing weight-loaded forced swimming further supported the beneficial effects of LicoB. Four weeks of oral administration of LicoB improved physical endurance, increased liver glycogen content, antioxidant enzyme activity in muscle, and blood free fatty acid level, while reducing circulating blood corticosterone and lactate levels, as well as lipid peroxidation in muscle tissues. Upregulation of TP53-induced regulator of glycolysis and apoptosis, sirtuin 1, and peroxisome proliferator-activated receptor-γ coactivator 1α in skeletal muscles suggested enhanced mitochondrial function and reduced oxidative damage. Additionally, transcriptomic analysis of skeletal muscle indicated that LicoB induced gene programs involved in myogenesis, neuromuscular junctions, and glucose metabolism. Collectively, these findings suggest that LicoB plays multi-targeted protective roles against excessive exercise-induced muscle fatigue by attenuating oxidative stress and modulating energy metabolism. Therefore, LicoB is a promising nutraceutical candidate for improving muscle endurance and recovery after strenuous physical activity.
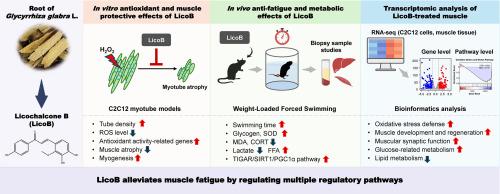
甘草查尔酮B通过其抗氧化活性减轻实验性小鼠运动性肌肉疲劳。
肌肉疲劳是体能表现的主要制约因素,由多种因素驱动,如过度氧化应激和ATP消耗。本研究研究了甘草查尔酮B (LicoB)对氧化应激诱导的C2C12细胞损伤的保护作用。此外,还对小鼠进行了竭力游泳的抗疲劳电位评价。在C2C12肌管中,LicoB通过清除自由基显著降低h2o2诱导的氧化损伤并保持肌管完整性。对C2C12细胞的转录组学分析显示,LicoB逆转了应激诱导的抗氧化和肌生成基因的失调,表明具有保护性的转录重编程。采用负重强迫游泳的小鼠体内模型进一步支持了LicoB的有益作用。口服4周的LicoB改善了身体耐力,增加了肝糖原含量、肌肉抗氧化酶活性和血液游离脂肪酸水平,同时降低了循环血液皮质酮和乳酸水平,以及肌肉组织脂质过氧化。tp53诱导的骨骼肌糖酵解和凋亡调节因子sirtuin 1和过氧化物酶体增殖体激活受体γ共激活因子1α的上调表明线粒体功能增强,氧化损伤减轻。此外,骨骼肌的转录组学分析表明,LicoB诱导的基因程序涉及肌肉形成、神经肌肉连接和葡萄糖代谢。综上所述,这些发现表明,LicoB通过减轻氧化应激和调节能量代谢,对过度运动引起的肌肉疲劳具有多靶点的保护作用。因此,LicoB是一种很有前途的营养候选物,可以提高肌肉耐力和剧烈运动后的恢复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


