SREBP1a induced PINK1-Parkin mediated mitophagy facilitates ovarian cancer progression
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-15
DOI:10.1016/j.bbadis.2025.168043
引用次数: 0
Abstract
Sterol regulatory element binding protein 1 (SREBP1) has emerged as a central regulator of lipid metabolism, playing a pivotal role in cancer progression. However, the oncogenic potential of SREBP1a is still underexplored. This study investigates the multifaceted contributions of SREBP1a on tumorigenesis, with a particular focus on ovarian cancer. Elevated expression of the SREBP1a isoform was found to enhance proliferation, migration, and invasion of ovarian cancer cells. Mechanistically, SREBP1a induces mitochondrial fission by upregulating DRP1 expression and promoting its activation through ser616 phosphorylation, resulting in a fragmented mitochondrial network that supports enhanced bioenergetic flexibility. In parallel, SREBP1a drives PINK1-Parkin-mediated mitophagy. This coupling of mitochondrial fission and mitophagy possibly ensures mitochondrial quality control, enhances cellular bioenergetics, and increases ATP production, supporting rapid cell proliferation and migration. Experimental evidences reveal that SREBP1 directly regulates DRP1 and PINK1 transcription, reinforcing its role in regulating mitochondrial dynamics. Furthermore, targeting SREBP1 using Fatostatin, a small-molecule inhibitor, effectively disrupts mitochondrial fission, impairs mitophagy, and attenuates tumor progression. These findings highlight the novel role of SREBP1a as a key regulator of mitochondrial dynamics, establishing it as a promising therapeutic target in ovarian cancer. Future studies should explore combinatorial strategies integrating SREBP1a inhibition with existing therapies to improve treatment outcomes.
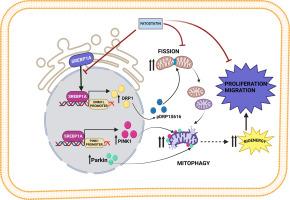
SREBP1a诱导的PINK1-Parkin介导的线粒体自噬促进卵巢癌进展
甾醇调节元件结合蛋白1 (SREBP1)是脂质代谢的中心调节因子,在癌症进展中起关键作用。然而,SREBP1a的致癌潜力仍未被充分发掘。本研究探讨了SREBP1a在肿瘤发生中的多方面贡献,特别关注卵巢癌。SREBP1a亚型的表达升高可增强卵巢癌细胞的增殖、迁移和侵袭。从机制上讲,SREBP1a通过上调DRP1表达并通过ser616磷酸化促进其激活来诱导线粒体分裂,从而导致线粒体网络碎片化,从而支持增强的生物能量灵活性。同时,SREBP1a驱动pink1 - parkin介导的有丝分裂。线粒体分裂和线粒体自噬的耦合可能确保线粒体质量控制,增强细胞生物能量,增加ATP的产生,支持细胞快速增殖和迁移。实验证据表明,SREBP1直接调控DRP1和PINK1的转录,加强了其在线粒体动力学中的调节作用。此外,使用Fatostatin(一种小分子抑制剂)靶向SREBP1,有效地破坏线粒体分裂,损害线粒体自噬,并减缓肿瘤进展。这些发现强调了SREBP1a作为线粒体动力学的关键调节因子的新作用,使其成为卵巢癌的一个有希望的治疗靶点。未来的研究应探索将SREBP1a抑制与现有疗法结合的组合策略,以改善治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


