Macelignan, a lignan from Myristica fragrans Houtt., rescues mitochondrial homeostasis and prevents cognitive decline in vascular dementia by modulating the mTOR-Mitophagy axis
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Myristica fragrans Houtt. has a long history of use in traditional medicine as a nervine tonic for enhancing cognitive function, relieving anxiety, and managing neurological symptoms associated with aging. Given its established ethnopharmacological profile, we postulated that its principal active lignan, Macelignan, could exert potent neuroprotective effects in the context of neurodegenerative diseases like vascular dementia (VaD).
Aim of the study
This study was designed to investigate the neuroprotective properties of Macelignan in a preclinical model of vascular dementia (VaD) and to elucidate the underlying molecular mechanisms, with a particular focus on its modulation of the mTOR signaling pathway and mitochondrial homeostasis.
Materials and methods
In this study, in vivo and in vitro models of ischemia-hypoxia were established in Wistar rats by bilateral common carotid artery occlusion (BCCAo) and in HT22 neuronal cells with cobalt chloride (CoCl2) treatment, respectively. The resulting cognitive, pathological, and cell survival damages were comprehensively assessed using a battery of behavioral tests, histological staining (H&E and Nissl), and the CCK-8 assay. To elucidate the underlying mechanisms, we first predicted and validated the direct interaction between the drug and its core target protein using transcriptome sequencing (RNA-seq) combined with molecular docking and dynamics simulations. Subsequently, changes in key signaling pathways, including mTOR, mitochondrial dynamics, autophagy, and apoptosis, were systematically investigated utilizing Seahorse metabolic flux analysis, transmission electron microscopy (TEM), various fluorescent probes (JC-1, MitoSOX, ROS, Ca2+), Western blotting, and immunofluorescence (IF). All data were statistically analyzed using GraphPad Prism 10.1.2.
Results
In vivo, we demonstrate that Macelignan ameliorates neuronal damage and cognitive deficits in a model of vascular dementia by directly targeting and activating the mTOR signaling pathway. At the cellular level, this mTOR activation orchestrates a multifaceted protective response, which includes restoring mitochondrial function and homeostasis, enhancing antioxidant defenses, suppressing the stress-induced expression of mitophagy-related proteins Beclin-1 and Parkin, and potently inhibiting apoptosis. Critically, these neuroprotective effects of Mace were completely abrogated by the mTORC1-specific inhibitor rapamycin, definitively establishing that its therapeutic efficacy is dependent on mTOR activation.
Conclusions
Macelignan targets and activates mTOR to restore mitochondrial homeostasis, thereby ameliorating vascular dementia.
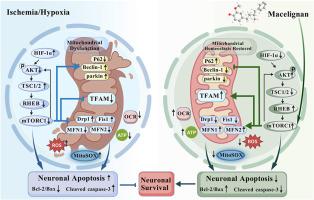
Macelignan,一种来自胡特肉豆蔻的木脂素。通过调节mTOR-Mitophagy轴,挽救线粒体稳态并防止血管性痴呆的认知能力下降
鸢尾草的民族药理学意义。在传统医学中作为神经滋补品用于增强认知功能、缓解焦虑和管理与衰老相关的神经系统症状有着悠久的历史。鉴于其已建立的民族药理学特征,我们假设其主要活性木脂素Macelignan可能在血管性痴呆(VaD)等神经退行性疾病的背景下发挥有效的神经保护作用。本研究旨在研究Macelignan在血管性痴呆(VaD)临床前模型中的神经保护特性,并阐明其潜在的分子机制,特别关注其对mTOR信号通路和线粒体稳态的调节。材料与方法本研究分别建立Wistar大鼠双侧颈总动脉闭塞(BCCAo)和HT22神经元细胞氯化钴(CoCl2)处理的体内和体外缺血-缺氧模型。通过一系列行为测试、组织学染色(H&;E和Nissl)和CCK-8测定,全面评估由此产生的认知、病理和细胞存活损伤。为了阐明潜在的机制,我们首先利用转录组测序(RNA-seq)结合分子对接和动力学模拟,预测并验证了药物与其核心靶蛋白之间的直接相互作用。随后,利用海马代谢通量分析、透射电子显微镜(TEM)、各种荧光探针(JC-1、MitoSOX、ROS、Ca2+)、Western blotting和免疫荧光(IF)系统地研究了mTOR、线粒体动力学、自噬和凋亡等关键信号通路的变化。所有数据采用GraphPad Prism 10.1.2进行统计分析。在体内,我们证明Macelignan通过直接靶向和激活mTOR信号通路改善血管性痴呆模型中的神经元损伤和认知缺陷。在细胞水平上,mTOR的激活协调了多方面的保护反应,包括恢复线粒体功能和稳态,增强抗氧化防御,抑制应激诱导的线粒体自噬相关蛋白Beclin-1和Parkin的表达,以及有效地抑制细胞凋亡。至关重要的是,mtorc1特异性抑制剂雷帕霉素完全消除了Mace的这些神经保护作用,从而明确表明其治疗效果依赖于mTOR的激活。结论马塞利木素靶向并激活mTOR恢复线粒体稳态,从而改善血管性痴呆。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


