Metabolite profiling in human and mouse liver microsomes of the aminosteroid RM-581, a new orally active anticancer agent
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
To progress towards clinical phase studies with orally active aminosteroid RM-581, it was necessary to address its metabolic stability. Analysis by ultra high-pressure liquid chromatography coupled with quadrupole time-of-flight-mass spectrometry (MS) showed the gradual disappearance of RM-581, making it possible to determine a half-life of 14 and 15 min in mouse and human liver microsomes, respectively, and the presence of 6 metabolites in varying proportions (M1 to M6). The MS/MS spectra of metabolites were investigated to assign sub-structures to fragment ions. These analyses made it possible to conclude that the amino acid proline in the C2-side chain of the steroid mestranol core was the main site of metabolism, and the following mass shift relative to the parent compound were observed for each metabolite relative to RM-581: M1(+34), M2(−2), M3(+16), M4(+18), M5(+32) and M6(+16). Suspecting the formation of RM-581 analogs whose pyrrolidine ring of proline has been opened, compound 8 (M4) was synthesized and characterized. Its chromatographic and MS properties made it possible to formally identify the metabolite M4 (M + H+ = 665.3684; Rt = 9.11 min) as a primary alcohol derivative. This result also indirectly supports the proposed structures of the other metabolites M6 (aldehyde), M5 (carboxylic acid) and M1 (dihydroxylated quinoline ring). M4 (IC50 = 12–454 μM) was found to be much less potent than RM-581 (IC50 = 0.78–7.55 μM) when tested on 9 human pancreas and leukemia cancer cell lines. M4 (63 %) was also more present in plasma than RM-581 (37 %) when RM-581 was orally administered to rats.
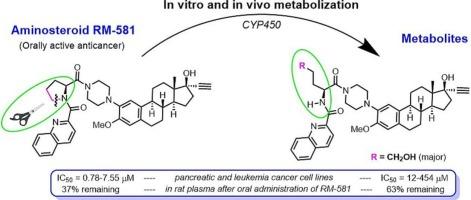
新型口服抗癌药物氨基类固醇RM-581在人和小鼠肝微粒体中的代谢物分析
为了进行口服活性氨基类固醇RM-581的临床研究,有必要解决其代谢稳定性问题。超高压液相色谱联用四极杆飞行时间质谱(MS)分析显示,RM-581逐渐消失,在小鼠和人肝微粒体中的半衰期分别为14和15分钟,并且存在6种不同比例的代谢物(M1至M6)。研究了代谢物的MS/MS谱,以确定片段离子的亚结构。这些分析可以得出结论,甾体美甾醇核心c2侧链上的氨基酸脯氨酸是代谢的主要部位,并且相对于RM-581,每个代谢产物相对于母体化合物的质量变化如下:M1(+34), M2(- 2), M3(+16), M4(+18), M5(+32)和M6(+16)。怀疑形成了脯氨酸吡咯烷环被打开的RM-581类似物,合成了化合物8 (M4)并对其进行了表征。其色谱和质谱性质使其代谢产物M4 (M + H+ = 665.3684; Rt = 9.11 min)可以正式鉴定为伯醇衍生物。这一结果也间接支持了其他代谢物M6(醛)、M5(羧酸)和M1(二羟基喹啉环)的结构。结果表明,M4 (IC50 = 12 ~ 454 μM)对9种人胰腺和白血病细胞的作用远低于RM-581 (IC50 = 0.78 ~ 7.55 μM)。当给大鼠口服RM-581时,血浆中M4(63%)的含量也高于RM-581(37%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


