Exploring the anticancer potential of β-amyrin: Scalable process for isolation, semi synthesis, and molecular docking studies
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
β-Amyrin, a pentacyclic triterpenoid with diverse biological activities, was efficiently isolated from Alstonia scholaris using a scalable and simplified process. To enhance its therapeutic potential, a series of C-3 modified semisynthetic derivatives bearing heterocyclic moieties were synthesized (3a-3o, 4a-4k, 6a-6h and 7a-7n). Several derivatives exhibited promising anticancer activity, notably through Bax-mediated apoptotic pathways. Molecular docking and simulation studies confirmed strong interactions with the Bax protein trigger site, supporting their proposed mechanism of action. These findings highlight the potential of β-amyrin derivatives as lead candidates for further development in anticancer drug discovery.
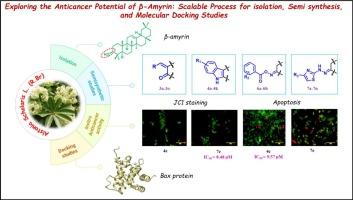
探索β-amyrin的抗癌潜力:可扩展的分离、半合成和分子对接研究过程
β-Amyrin是一种具有多种生物活性的五环三萜类化合物,采用可扩展和简化的工艺从雪桐中高效分离得到。为了提高其治疗潜力,我们合成了一系列含杂环基团的C-3修饰半合成衍生物(3a- 30,4a -4k, 6a-6h和7a-7n)。一些衍生物显示出有希望的抗癌活性,特别是通过bax介导的凋亡途径。分子对接和模拟研究证实了与Bax蛋白触发位点的强相互作用,支持了它们提出的作用机制。这些发现突出了β-amyrin衍生物作为进一步开发抗癌药物的主要候选物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


