Oocyte-specific Ahr deletion disrupts folliculogenesis and female fertility in mice
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that regulates various biological processes, including xenobiotic metabolism, immune response, and reproduction. Although previous studies have shown that AHR plays a role in ovarian follicle development, the precise role of oocyte-expressed AHR in female reproduction remains unclear. In this study, oocyte-specific Ahr knockout (cKO) mice generated by crossing the Ahr flox/flox (Ahr fl/fl) and Gdf9-cre transgenic mouse strains were used to answer this open question. The cKO female mice exhibited a disrupted estrous cyclicity and subfertility. Histological analyses demonstrated that oocyte AHR loss reduces the number of primary follicles while increasing the number of secondary follicles and corpus lutea in mouse ovary. Hormonal analysis revealed decreased serum estradiol and follicle-stimulating hormone, indicating a disruption of the hypothalamic-pituitary-gonadal axis in cKO mice. TUNEL and Western blotting results demonstrate that deletion of oocyte AHR also results in increased apoptosis in ovarian granulosa cells (GCs), downregulated expression of Gdf9 and Bmp15 in oocytes, and disrupted bidirectional oocyte-GC communication. In conclusion, our findings reveal that the aryl hydrocarbon receptor plays a role beyond sensing environmental chemicals and endogenous compounds and underscore a critical role of oocyte-expressed Ahr in maintain follicle development, ovarian function, and female reproductive health.
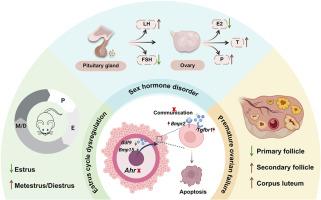
卵母细胞特异性Ahr缺失破坏小鼠卵泡发生和雌性生育能力。
芳烃受体(AHR)是一种配体激活的转录因子,调节多种生物过程,包括异种代谢、免疫反应和生殖。虽然先前的研究表明AHR在卵巢卵泡发育中起作用,但卵母细胞表达的AHR在女性生殖中的确切作用尚不清楚。本研究利用Ahrflox/flox (Ahrfl/fl)和Gdf9-cre转基因小鼠杂交产生的卵母细胞特异性Ahr敲除(cKO)小鼠来回答这个开放性问题。cKO雌性小鼠表现出发情周期中断和生育能力低下。组织学分析表明,卵母细胞AHR缺失减少了小鼠卵巢初级卵泡的数量,增加了次级卵泡和黄体的数量。激素分析显示血清雌二醇和促卵泡激素下降,表明cKO小鼠的下丘脑-垂体-性腺轴受到破坏。TUNEL和Western blotting结果表明,卵母细胞AHR的缺失也导致卵巢颗粒细胞(GCs)凋亡增加,下调卵母细胞Gdf9和Bmp15的表达,破坏卵母细胞与gc的双向通讯。总之,我们的研究结果揭示了芳烃受体在感知环境化学物质和内源性化合物之外的作用,并强调了卵母细胞表达的Ahr在维持卵泡发育、卵巢功能和女性生殖健康方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


