Targeting prokaryotic ion channel by a chimera of fluorescent protein and artificial peptide toxin
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
KcsA, a potassium channel from Streptomyces lividans, is one of the most extensively studied transmembrane proteins. Despite significant research in structural biology, relatively few ligands of KcsA have been identified. One such ligand is Hui1, an artificial peptide derived from a phage display screening using a combinatorial library constructed relying on several sea anemone toxins. In this study, we engineered a fluorescent probe by fusing Hui1 with enhanced green fluorescent protein (eGFP), creating the first fluorescence-based tool to visualize prokaryotic ion channels. The eGFP–Hui1 chimera was successfully produced in Escherichia coli and purified using chromatographic techniques. Our study revealed a direct interaction between KcsA, also recombinantly expressed in E. coli, and the fluorescent chimera. Furthermore, we demonstrated that both Hui1 and tetraethylammonium can effectively displace the chimera from its complex with KcsA, confirming the specificity of the binding interaction. This approach opens new avenues for pharmacological and structural investigations, including the development of novel antimicrobial agents and high-throughput ligand screening.
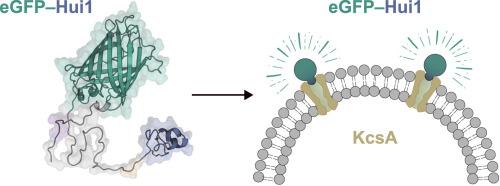
荧光蛋白与人工肽毒素嵌合体靶向原核离子通道。
KcsA是一种来自lividans链霉菌的钾离子通道,是研究最广泛的跨膜蛋白之一。尽管在结构生物学方面进行了大量的研究,但已经确定的KcsA配体相对较少。其中一种配体是Hui1,它是一种人工肽,来源于噬菌体展示筛选,使用依赖几种海葵毒素构建的组合文库。在这项研究中,我们设计了一个荧光探针,将Hui1与增强型绿色荧光蛋白(eGFP)融合,创造了第一个基于荧光的工具来可视化原核离子通道。在大肠杆菌中成功地产生了eGFP-Hui1嵌合体,并用色谱技术对其进行了纯化。我们的研究揭示了在大肠杆菌中重组表达的KcsA与荧光嵌合体之间的直接相互作用。此外,我们证明了Hui1和四乙基铵都能有效地将嵌合体从其与KcsA的配合物中置换出来,证实了结合相互作用的特异性。这种方法为药理学和结构研究开辟了新的途径,包括开发新的抗菌药物和高通量配体筛选。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


