Spotlight on membrane fluidity of normal and cancer cells: Implications for cancer diagnosis and treatment
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Membrane fluidity is crucial for cellular function, signalling, and adaptation. Healthy cells maintain strict control over membrane fluidity through homeostatic mechanisms such as lipid-protein interactions, phospholipid asymmetry and cholesterol distribution. In contrast, cancer cells exhibit profound dysregulation. Altered lipid metabolism, increased incorporation of unsaturated fatty acids and disrupted cholesterol homeostasis create a more fluid and dynamic membrane environment, thereby enhancing oncogenic signalling, mechanotransduction and immune evasion. These changes are key drivers of cancer behaviours, including enhanced proliferation, resistance to apoptosis and metastasis.
This review explores the biophysical basis of membrane fluidity, examining its dual role as a diagnostic biomarker and a therapeutic target in cancer. Advanced imaging techniques, such as fluorescence recovery after photobleaching (FRAP), fluorescence lifetime imaging microscopy (FLIM) and electron spin resonance (ESR), enable precise measurement of membrane fluidity, revealing cancer-specific alterations. These tools provide high-resolution insights into lipid organization and protein mobility, facilitating improved cancer diagnostics and therapeutic response monitoring. Emerging therapeutic strategies exploit membrane fluidity to selectively induce cancer cell death. These strategies include modulating cholesterol levels, using lipid metabolism inhibitors, and activating sonosensitisers, intracellular responsive chemical agents that generate reactive oxygen species, using ultrasound by sonodynamic therapy.
Despite these advances, there are still challenges in translating membrane-targeted strategies into clinical practice, primarily due to tumour heterogeneity and the complex relationship between lipid dynamics and protein function. Future research must integrate lipidomics, biophysics, and oncology to exploit membrane fluidity as a biomarker and therapeutic target, paving the way for more precise cancer treatments.
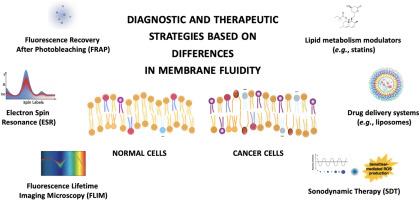
聚焦于正常细胞和癌细胞的膜流动性:对癌症诊断和治疗的意义
膜流动性对细胞功能、信号传导和适应至关重要。健康细胞通过脂质-蛋白相互作用、磷脂不对称和胆固醇分布等稳态机制严格控制细胞膜流动性。相反,癌细胞表现出严重的失调。脂质代谢的改变、不饱和脂肪酸的增加和胆固醇稳态的破坏创造了一个更加流动和动态的膜环境,从而增强了致癌信号、机械转导和免疫逃避。这些变化是癌症行为的关键驱动因素,包括增强增殖、抵抗细胞凋亡和转移。本文综述了膜流动性的生物物理基础,探讨了其作为诊断生物标志物和癌症治疗靶点的双重作用。先进的成像技术,如光漂白后荧光恢复(FRAP)、荧光寿命成像显微镜(FLIM)和电子自旋共振(ESR),能够精确测量膜流动性,揭示癌症特异性改变。这些工具提供了对脂质组织和蛋白质迁移的高分辨率见解,促进了癌症诊断和治疗反应监测的改进。新兴的治疗策略利用膜的流动性来选择性地诱导癌细胞死亡。这些策略包括调节胆固醇水平,使用脂质代谢抑制剂,激活声敏剂,细胞内反应性化学剂产生活性氧,使用超声声动力治疗。尽管取得了这些进展,但由于肿瘤的异质性以及脂质动力学和蛋白质功能之间的复杂关系,将膜靶向策略转化为临床实践仍然存在挑战。未来的研究必须将脂质组学、生物物理学和肿瘤学结合起来,利用膜流动性作为生物标志物和治疗靶点,为更精确的癌症治疗铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


