KRAS mutations promote PD-L1-mediated immune escape by ETV4 in lung adenocarcinoma
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
KRAS mutations are frequently associated with immune escape in lung adenocarcinoma. The aim of this research is to investigate the molecular mechanism underlying the KRAS-driven upregulation of PD-L1 and its role in immune escape. Methods: Transcriptomic data combined with data from the GEO database and immunohistochemistry were used to analyze the expression of PD-L1 in KRAS-mutant tissues. Functional experiments were performed using KRAS knockdown and MEK-ERK signaling pathway inhibitors to reveal the major signaling pathways by which KRAS mutations regulate PD-L1 expression. The key transcription factors regulating PD-L1 expression were identified through weighted gene coexpression network analysis (WGCNA) combined with dataset screening, and the binding sites of the key transcription factors to the PD-L1 promoter region were predicted using the JASPAR database and verified by luciferase reporting experiments and ChIP experiments. Flow cytometry, LDH assays, graft tumor assays, multicolor immunofluorescence and immunohistochemistry were used to determine whether key transcription factors affected PD-L1-mediated immune escape in KRAS-mutated lung adenocarcinoma. Results: PD-L1 expression was markedly increased in KRAS-mutant lung adenocarcinoma, and the MEK-ERK signaling pathway was identified as the main pathway promoting the upregulation of PD-L1. KRAS mutations promoted PD-L1 expression through the key transcription factor ETV4, which binds to specific sequences in the promoter region of PD-L1 to directly regulate its expression. KRAS mutations promoted PD-L1-mediated immune escape by ETV4. Conclusion: Carcinogenic KRAS mutations in lung adenocarcinoma regulate PD-L1 expression mainly through the MEK-ERK-ETV4 signaling axis. ETV4, as a transcription factor, activates PD-L1 expression and promotes immune escape in KRAS-mutant lung adenocarcinoma.
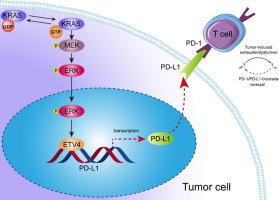
KRAS突变促进肺腺癌中pd - l1介导的ETV4免疫逃逸
KRAS突变通常与肺腺癌的免疫逃逸有关。本研究的目的是探讨kras驱动的PD-L1上调的分子机制及其在免疫逃逸中的作用。方法:利用转录组学数据、GEO数据库数据和免疫组织化学分析kras突变组织中PD-L1的表达。通过KRAS敲低和MEK-ERK信号通路抑制剂进行功能实验,揭示KRAS突变调节PD-L1表达的主要信号通路。通过加权基因共表达网络分析(WGCNA)结合数据集筛选确定调节PD-L1表达的关键转录因子,利用JASPAR数据库预测关键转录因子与PD-L1启动子区域的结合位点,并通过荧光素酶报告实验和ChIP实验进行验证。采用流式细胞术、LDH检测、移植物肿瘤检测、多色免疫荧光和免疫组织化学检测关键转录因子是否影响kras突变肺腺癌中pd - l1介导的免疫逃逸。结果:kras突变肺腺癌中PD-L1表达明显升高,MEK-ERK信号通路被确定为促进PD-L1上调的主要通路。KRAS突变通过关键转录因子ETV4促进PD-L1表达,ETV4结合PD-L1启动子区域的特定序列,直接调控PD-L1的表达。KRAS突变通过ETV4促进pd - l1介导的免疫逃逸。结论:肺腺癌的致癌KRAS突变主要通过MEK-ERK-ETV4信号轴调控PD-L1的表达。在kras突变型肺腺癌中,ETV4作为转录因子激活PD-L1表达,促进免疫逃逸。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


