Personalizing colorectal Cancer treatment: Chemotherapy drug testing with patient-derived tumor cell clusters
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
Due to the heterogeneity of colorectal cancer (CRC), models that can predict the chemotherapy response are needed to facilitate personalized treatment.
Aim
To construct patient-derived tumor-like cell cluster (PTC) models in vitro drug sensitivity screening for CRC personalized chemotherapy.
Methods
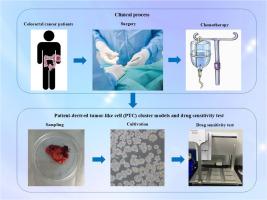
We collected 140 CRC tissues via surgical resection in three Chinese hospitals and establish PTC models which is highly similar to the original tumor tissue. The sensitivity to various chemotherapy drugs was assessed in these PTC models. We recorded the PTC model cultivation process and patients' clinical data and assessed the concordance between in vitro drug sensitivity and clinical outcomes.
Results
PTC models were successfully established from 124 specimens, with a success rate of 88.6 %. The average culture time was 3.02 ± 1.56 days, and the median time to obtain drug sensitivity results was 11 days (10–13 days). Drug sensitivity testing revealed that the PTC models had variable responses to different chemotherapy regimens, with some patients showing unexpected sensitivity to regimens not typically considered first-line treatments. The median follow-up time for all patients was 19 months, and there was no significant difference in disease-free survival (DFS) between patients whose actual responses to clinical treatment regimens were consistent or inconsistent with the PTC model predictions.
Conclusion
The PTC model for drug sensitivity testing has advantages of high success rate and rapid drug screening time. This study provides a promising tool for personalized chemotherapy sensitivity screening in patients with CRC and, after further clinical trials, may guide clinical treatment decision making.
个体化大肠癌治疗:患者源性肿瘤细胞簇的化疗药物试验
由于结直肠癌(CRC)的异质性,需要能够预测化疗反应的模型来促进个性化治疗。目的构建患者源性肿瘤样细胞簇(PTC)模型用于结直肠癌个体化化疗的体外药物敏感性筛选。方法收集国内3家医院手术切除的140例结直肠癌组织,建立与原肿瘤组织高度相似的PTC模型。在这些PTC模型中评估对各种化疗药物的敏感性。记录PTC模型培养过程及患者临床资料,评估体外药敏与临床结果的一致性。结果124例标本成功建立sptc模型,成功率为88.6%。平均培养时间为3.02±1.56 d,获得药敏结果的中位时间为11 d (10 ~ 13 d)。药物敏感性测试显示,PTC模型对不同的化疗方案有不同的反应,一些患者对通常不被认为是一线治疗的方案表现出意想不到的敏感性。所有患者的中位随访时间为19个月,对临床治疗方案的实际反应与PTC模型预测一致或不一致的患者的无病生存期(DFS)无显著差异。结论采用PTC模型进行药敏试验具有成功率高、筛选时间短等优点。本研究为CRC患者的个性化化疗敏感性筛查提供了一个有希望的工具,并在进一步的临床试验后可能指导临床治疗决策。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


