Synthesis, Characterization, Antimicrobial Activity and Molecular Docking Studies of Novel Pyrano[2,3-c]Pyrazole Derivatives
IF 2.6
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Antimicrobial activity is one of the most critical functional properties of β-enaminonitriles, pyranopyrazoles, and pyrazolopyranopyrimidines since bacteria and fungi very quickly attack these heterocycles. Therefore, a series of novel β-enaminonitriles (3a–d) were constructed by different pathways. An efficient MCR of these derivatives involving β-ketoesters, 2,4-dinitrophenylhydrazine, malononitrile, and aromatic aldehydes using different catalysts under reaction conditions with excellent yields is established. The β-enaminonitrile (3a) was used as a key intermediate for the synthesis of heterocycles as pyrazolopyranopyrimidines (4, 6) and pyranopyrazoles (5, 7, and 8). The chemical structures were confirmed through elemental analysis and spectral data as FT-IR,1H-NMR,13C-NMR, and mass spectra. The antibacterial activity of the synthesized compounds has been tested against Gram-positive bacteria (Bacillus cereus, Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa). The compounds (3a, 3b, and 3d) showed good antibacterial activity, but compound (3c) displayed a potent high inhibitory activity Additionally, the antifungal activity has been tested against Aspergillus niger, Penicillium sp. and Candida albicans, the compound (3a, 3b, 3d, 4, and 6) showed good antifungal activity. The potential inclusion of nitro substituents may undoubtedly increase the activity of these compounds. Based on the inhibition zone determination, the results indicated that these novel heterocycles improve their antimicrobial activity. The molecular docking was studied, Tyrosyl-tRNA synthetase binding affinities and viable interaction modes were rationalized using a molecular docking study conducted against S. aureus bacteria.
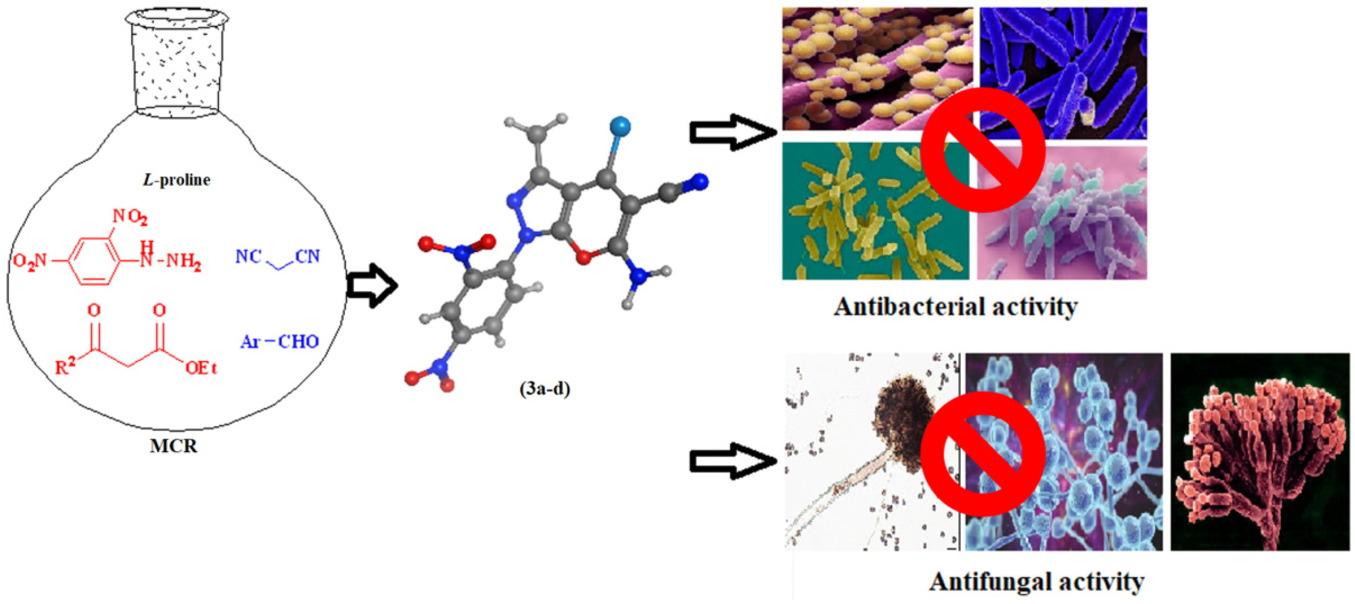
新型吡喃[2,3-c]吡唑衍生物的合成、表征、抗菌活性及分子对接研究
抗菌活性是β-氨基腈、吡喃吡唑和吡喃吡嘧啶最关键的功能特性之一,因为细菌和真菌非常迅速地攻击这些杂环。因此,通过不同途径构建了一系列新的β-氨基腈(3a-d)。在不同的催化剂条件下,建立了β-酮酯、2,4-二硝基苯肼、丙二腈和芳香醛类衍生物的高效MCR反应。β-氨基腈(3a)作为关键中间体,用于合成杂环化合物吡唑吡喃嘧啶(4,6)和吡唑吡喃嘧啶(5,7和8)。通过元素分析、FT-IR、1H-NMR、13C-NMR和质谱等光谱数据证实了化合物的化学结构。合成的化合物对革兰氏阳性菌(蜡样芽孢杆菌、金黄色葡萄球菌)和革兰氏阴性菌(大肠杆菌、铜绿假单胞菌)的抑菌活性进行了测试。化合物(3a、3b、3d)表现出良好的抑菌活性,其中化合物(3c)表现出较强的高抑菌活性。此外,化合物(3a、3b、3d、4、6)对黑曲霉、青霉和白色念珠菌的抑菌活性也进行了测试,化合物(3a、3b、3d、4、6)表现出较好的抑菌活性。硝基取代基的潜在包含无疑会增加这些化合物的活性。抑菌区测定结果表明,这些新型杂环化合物提高了其抑菌活性。对分子对接进行研究,通过对金黄色葡萄球菌的分子对接研究,理顺tyroyl - trna合成酶的结合亲和力和可行的相互作用模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


