Design and Synthesis of New Quinazolinone Derivatives Conjugated with Chalcone or Pyridazine Moieties: Anticancer Activities and Apoptotic Induction
IF 2.6
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
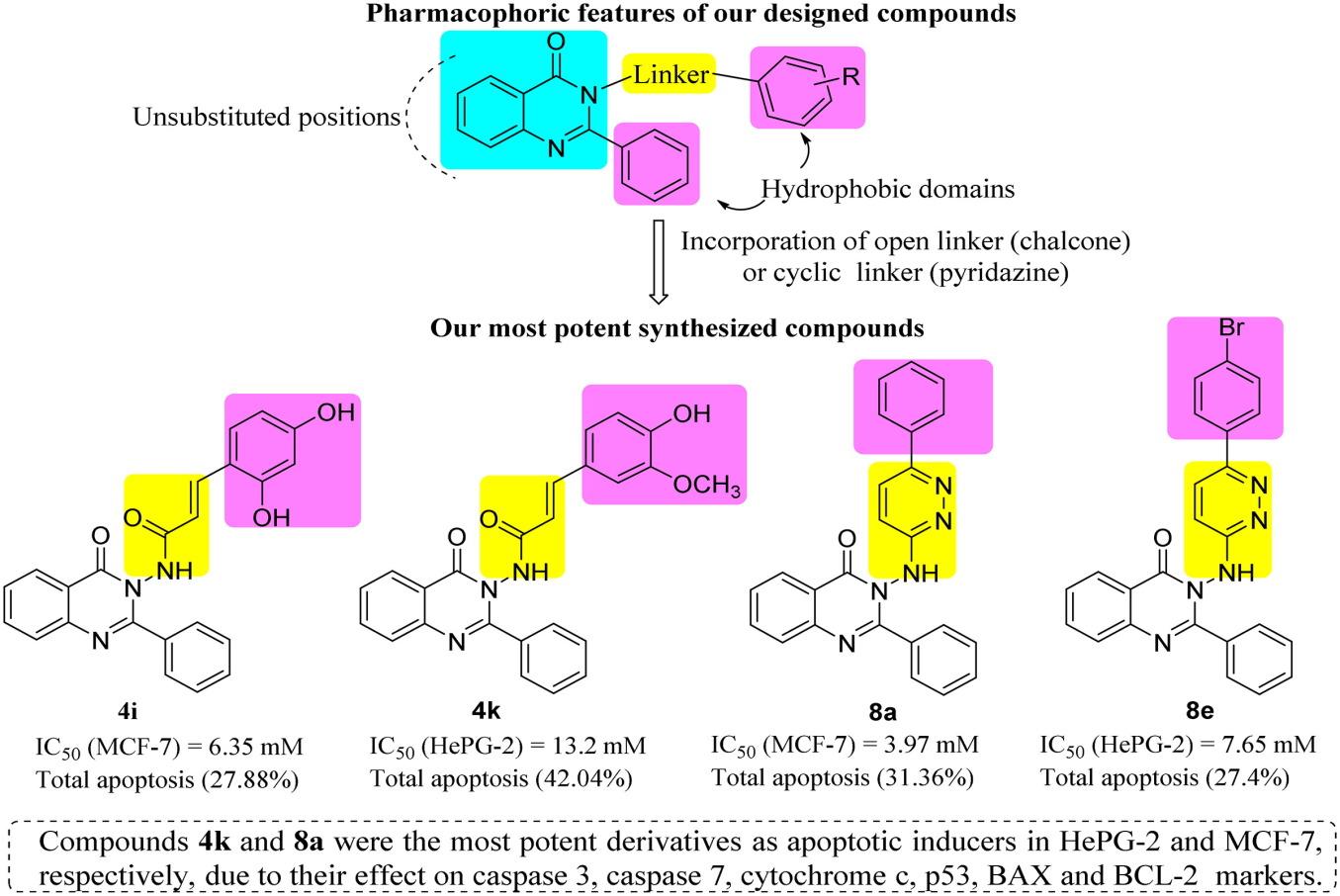
Two new series of quinazolinone derivatives, conjugated with chalcone 4a–k and pyridazine 8a–f moieties, were designed and synthesized as promising anticancer agents and apoptotic inducers. Using MTT assay, they were assessed for their cytotoxicity against HepG-2, HCT-116, and MCF-7 cancer cell lines and normal cell lines, compared to Doxorubicin as a reference drug. Quinazolinone-Pyridazinone hybrid 8a exhibited the highest cytotoxicity against all examined cancer cells and was safe against normal cells. Compounds 4i, 4k, and 8e showed good cytotoxicity against the cancer cell lines. The four most potent compounds were then screened for their effect on cell cycle distribution and apoptosis induction. Compounds 4i and 8a led to a cell cycle arrest at the G1 phase in MCF-7 cancer cells, while compounds 4k and 8e led to cell cycle arrest of HepG-2 cancer cells at the G1 phase and G2/M phase, respectively. The four hybrids induced total apoptosis which was proved via testing their effect on different apoptotic markers. They were further docked into a BCL-2 binding pocket showing good binding interactions. Therefore, the new quinazolinone derivatives might be identified as promising apoptotic inducers and can be used as lead compounds in the future investigations.
新型查尔酮或吡啶偶联喹唑啉酮衍生物的设计与合成:抗癌活性和诱导细胞凋亡
设计并合成了两个新系列的喹唑啉酮衍生物,它们分别与查尔酮4a-k和吡嗪8a-f基团偶联,是很有前途的抗癌药物和凋亡诱导剂。使用MTT法,与阿霉素作为对照药物相比,评估了它们对HepG-2、HCT-116和MCF-7癌细胞系和正常细胞系的细胞毒性。喹唑啉酮-吡嗪酮杂种8a对所有检测的癌细胞表现出最高的细胞毒性,对正常细胞是安全的。化合物4i、4k和8e对癌细胞具有良好的细胞毒性。然后筛选四种最有效的化合物对细胞周期分布和诱导凋亡的影响。化合物4i和8a导致MCF-7癌细胞的细胞周期阻滞在G1期,而化合物4k和8e分别导致HepG-2癌细胞的细胞周期阻滞在G1期和G2/M期。通过对不同细胞凋亡标志物的检测,证实了这一结论。它们进一步被对接到BCL-2结合口袋中,显示出良好的结合相互作用。因此,新的喹唑啉酮衍生物可能被确定为有前途的凋亡诱导剂,并可作为未来研究的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


