Synthesis and Characterization of Novel Benzothiazinonic N-Acylhydrazone Derivatives
IF 2.6
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Several recent research studies have demonstrated that N-acylhydrazones are well known as privileged scaffolds frequently used in the discovery of new potential antiparasitic compounds. The investigation of literature revealed that the synthesis of N˗acylhydrazones bearing the 1,4˗benzothiazin˗3-one pharmacophore has not been described. Therefore, it was considered interesting to attempt the synthesis of new N˗acylhydrazone derivatives containing 1,4˗benzothiazine˗3˗one fragment, thus obtaining a series of novel (E)-N′-(substituted benzylidene)-2(3-oxo-2H-benzo[b][1,4]thiazin-4(3H)-yl)acetohydrazides. Thus, the targeted compounds were successfully synthesized via an easy and general procedure. Hence, 2-aminothiophenol was reacted with maleic anhydride to produce the ester 3,4-dihydro-2-methoxycarbonylmethyl-3-oxo-2H-1,4-benzothiazine. The hydrazinolysis of the obtained ester-based benzothiazinon-3-one was also realized to give the corresponding benzothiazinonic acid hydrazide derivative as a precursor for the synthesis of the desired N˗acylhydrazones, by their condensation with various substituted benzaldehydes. In an attempt to explain the duplication of some peaks observed from the 1H and 13C˗NMR spectral analysis performed on the structures of all newly synthesized N˗acylhydrazones in DMSO˗d6; it was concluded that they exist as a mixture of syn˗E and anti˗E diastereoisomers with different isomeric yield ratio. Generally, the results obtained in this study indicate that these N˗acylhydrazones may be envisaged for supplementary structural investigations, and as potential biologically active compounds in diverse applications.
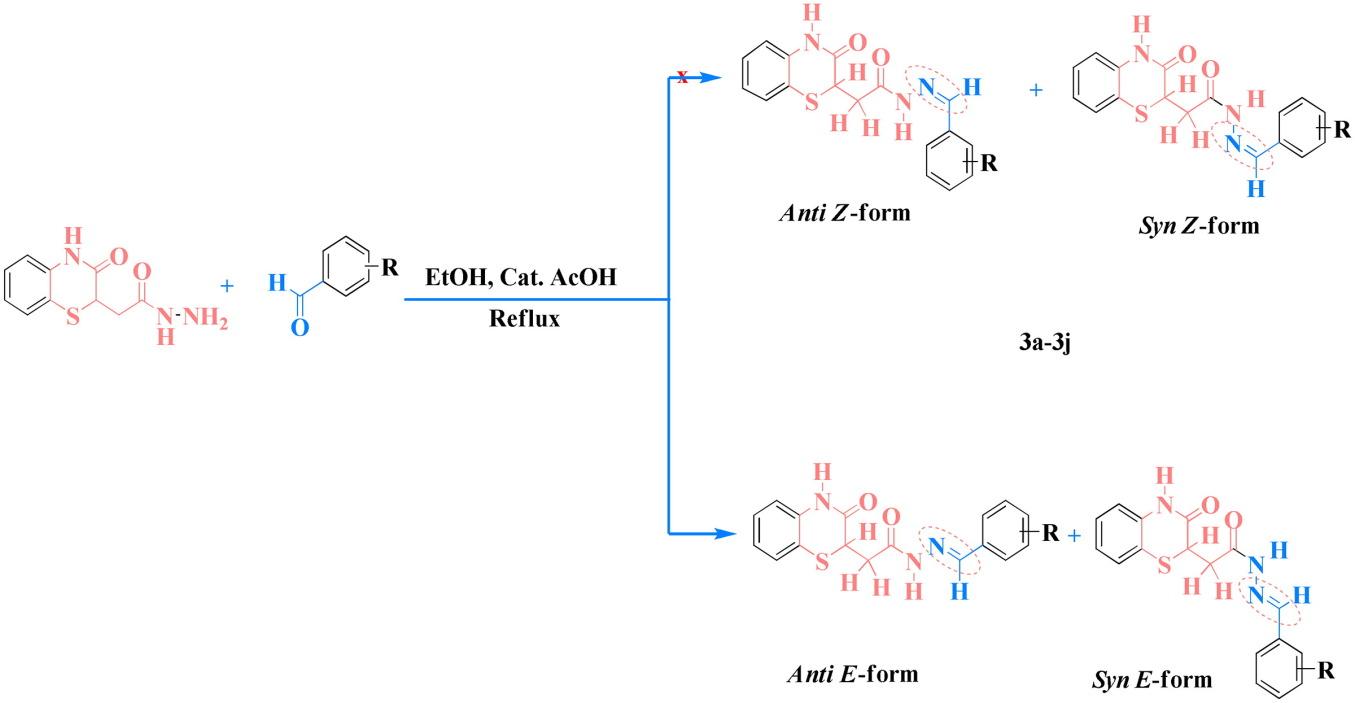
新型苯并噻唑基n -酰基腙衍生物的合成与表征
最近的几项研究表明,n -酰基腙是众所周知的特权支架,经常用于发现新的潜在抗寄生虫化合物。通过文献调查发现,含有1,4个药效团的N个腈基腙的合成尚未见文献记载。因此,我们有必要尝试合成含有1,4个氨基苄噻嗪的新型N -氨基腙衍生物,从而获得一系列新的(E)-N ' -(取代苄基)-2(3-氧- 2h -苯并[b][1,4]噻嗪-4(3H)-基)乙酰肼。因此,通过简单和一般的程序成功地合成了目标化合物。因此,2-氨基噻吩与马来酸酐反应生成3,4-二氢-2-甲氧基羰基甲基-3-氧- 2h -1,4-苯并噻嗪酯。得到的酯基苯并噻唑-3-酮通过与不同取代苯甲醛缩合得到相应的苯并噻唑酸肼衍生物,作为合成所需的N -嘧啶腙的前体。为了解释在DMSO中对所有新合成的N -酰腙进行1H和13C NMR分析时观察到的某些峰的重复;结果表明,它们是以不同产率的正、反两种对映异构体的混合物存在的。总的来说,本研究的结果表明,这些N -嘧啶腙可以作为补充结构研究的设想,并作为潜在的生物活性化合物在不同的应用中得到应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


