Artesunate induces ferroptosis in gastric cancer by targeting the TFRC-HSPA9 axis for iron homeostasis regulation
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ferroptosis, a recently characterized form of regulated cell death driven by iron-dependent lipid peroxidation, has emerged as a promising therapeutic strategy for cancer treatment due to its potential for selectively targeting cancer cells. Exploiting FDA-approved drugs to induce ferroptosis offers a novel approach that exploits cancer cells' vulnerabilities in iron metabolism and oxidative stress. Here, we identify artesunate, an antimalarial drug, as a potent inducer of ferroptosis in gastric cancer cells and reveal the transferrin receptor (TFRC) as a key mediator in this process. Notably, our study is the first to demonstrate an interaction between artesunate and TFRC through molecular docking and surface plasmon resonance (SPR) experiments, highlighting a novel mechanism by which artesunate stabilizes TFRC by inhibiting its lysosomal degradation. This stabilization is regulated via the heat shock protein HSPA9, another previously unreported interaction. Disrupting the TFRC-HSPA9 interaction facilitates iron accumulation and lipid peroxidation, hallmark features of ferroptosis, leading to significant cancer cell death. Additionally, in vivo studies confirm artesunate's anti-tumor efficacy, showing marked tumor growth inhibition and minimal systemic toxicity. These findings underscore the therapeutic relevance of targeting ferroptosis in cancer, particularly by leveraging TFRC's role in iron homeostasis. Furthermore, this study expands the understanding of post-translational regulation in ferroptosis, offering a new perspective on the role of artesunate in cancer therapy.
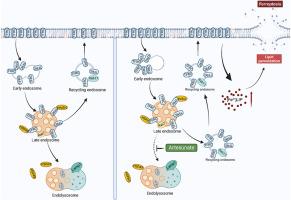
青蒿琥酯通过靶向TFRC-HSPA9轴调控铁稳态诱导胃癌铁下垂
铁凋亡是一种由铁依赖性脂质过氧化作用驱动的细胞死亡的新特征,由于其选择性靶向癌细胞的潜力,已成为一种有前途的癌症治疗策略。利用fda批准的药物诱导铁下垂提供了一种利用癌细胞在铁代谢和氧化应激中的脆弱性的新方法。本研究中,我们发现抗疟药物青蒿琥酯是胃癌细胞铁凋亡的有效诱导剂,并揭示了转铁蛋白受体(TFRC)在这一过程中的关键中介作用。值得注意的是,我们的研究首次通过分子对接和表面等离子体共振(SPR)实验证明了青蒿琥酯与TFRC之间的相互作用,强调了青蒿琥酯通过抑制其溶酶体降解来稳定TFRC的新机制。这种稳定性是通过热休克蛋白HSPA9调节的,这是另一种以前未报道的相互作用。破坏TFRC-HSPA9相互作用促进铁积累和脂质过氧化,这是铁下垂的标志特征,导致显著的癌细胞死亡。此外,体内研究证实了青蒿琥酯的抗肿瘤功效,显示出明显的肿瘤生长抑制和最小的全身毒性。这些发现强调了靶向铁下垂在癌症中的治疗相关性,特别是通过利用TFRC在铁稳态中的作用。此外,本研究扩展了对铁下垂翻译后调控的理解,为青蒿琥酯在癌症治疗中的作用提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


