Fat mass and obesity-associated protein inhibits macrophage-mediated inflammation via the m6A demethylation of c-Jun in COPD
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Fat mass and obesity-associated protein (FTO), which is a key regulator of N6-methyladenosine (m6A) RNA modification, is associated with inflammatory processes. Chronic obstructive pulmonary disease (COPD) is a prevalent inflammatory disease that affects airways. However, the precise mechanism underlying the FTO-mediated regulation of inflammation in COPD remains unclear. This study aimed to investigate the molecular mechanisms through which FTO-mediated m6A RNA demethylation regulates macrophage-driven inflammation in COPD. Bioinformatics analysis of a GEO dataset (GSE148004) and validation in lung tissues revealed significant downregulation of FTO expression in patients with COPD (n = 10). Consistent with these findings, decreased FTO protein levels and a significant increase in global m6A methylation were observed in CSE-stimulated U937-derived macrophages (n = 3) and alveolar macrophages from mice with COPD (n = 6). Additionally, FTO overexpression attenuated CSE-induced IL-6 and TNF-α production by U937-derived macrophages (n = 3), and this overexpression alleviated emphysematous changes and airway inflammation in mice with COPD (n = 6). Moreover, RNA sequencing analysis revealed c-Jun as a downstream target of FTO. Mechanistically, FTO suppressed the m6A modification of c-Jun mRNA, leading to increased c-Jun mRNA degradation, thereby attenuating macrophage-mediated inflammatory responses (n = 3). Thus, FTO negatively regulates macrophage-driven inflammation in COPD by promoting the m6A demethylation and destabilization of c-Jun mRNA. These findings indicate that FTO may represent a promising therapeutic target for mitigating inflammation in patients with COPD.
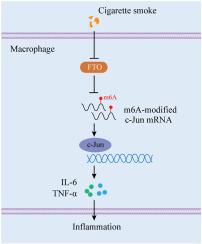
脂肪量和肥胖相关蛋白通过COPD中c-Jun的m6A去甲基化抑制巨噬细胞介导的炎症
脂肪量和肥胖相关蛋白(FTO)是n6 -甲基腺苷(m6A) RNA修饰的关键调节因子,与炎症过程有关。慢性阻塞性肺疾病(COPD)是一种影响气道的常见炎症性疾病。然而,fto介导的COPD炎症调节的确切机制尚不清楚。本研究旨在探讨fto介导的m6A RNA去甲基化调控COPD中巨噬细胞驱动炎症的分子机制。GEO数据集(GSE148004)的生物信息学分析和肺组织验证显示,COPD患者FTO表达显著下调(n = 10)。与这些发现一致,在cse刺激的u937来源的巨噬细胞(n = 3)和COPD小鼠的肺泡巨噬细胞(n = 6)中观察到FTO蛋白水平下降和总体m6A甲基化显著增加。此外,FTO过表达可减弱se诱导的u937源性巨噬细胞IL-6和TNF-α的产生(n = 3),并且这种过表达可减轻COPD小鼠的肺气肿变化和气道炎症(n = 6)。此外,RNA测序分析显示c-Jun是FTO的下游靶点。机制上,FTO抑制c-Jun mRNA的m6A修饰,导致c-Jun mRNA降解增加,从而减轻巨噬细胞介导的炎症反应(n = 3)。因此,FTO通过促进m6A去甲基化和c-Jun mRNA的不稳定来负性调节COPD中巨噬细胞驱动的炎症。这些发现表明,FTO可能是缓解COPD患者炎症的一个有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


