VDAC1 oligomerization-mediated mtDNA release under sublethal oxidative stress: A novel inflammatory mechanism in vitiligo
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Oxidative stress is a critical initiating factor in vitiligo, yet the early molecular events linking redox imbalance to melanocyte immune activation remain unclear. Here, we demonstrate that sub-lethal hydrogen peroxide (H2O2, 0.1 mM) exposure in human epidermal melanocytes induces a robust pro-inflammatory response independent of apoptosis or pyroptosis. This response is driven by the selective cytosolic release of mitochondrial DNA (mtDNA), which activates the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway. Mechanistically, voltage-dependent anion channel 1 (VDAC1) oligomerization cooperates with mitochondrial permeability transition pore (mPTP) opening to mediate mtDNA release. Both genetic VDAC1 knockdown and pharmacological inhibition blocked mtDNA leakage and downstream cytokine production. In H2O2-induced vitiligo mice, intradermal administration of the VDAC1 oligomerization inhibitor VBIT-4 restored melanin pigmentation, reduced CD8+ T cell infiltration, and alleviated cutaneous inflammation. These findings identify VDAC1-dependent mtDNA release as a key driver of innate immune activation in melanocytes and highlight VDAC1 as a potentially druggable therapeutic target for early intervention in vitiligo.
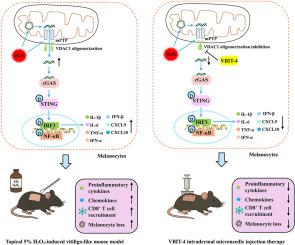
亚致死氧化应激下VDAC1寡聚化介导的mtDNA释放:白癜风中一种新的炎症机制
氧化应激是白癜风的一个关键启动因素,但将氧化还原失衡与黑素细胞免疫激活联系起来的早期分子事件尚不清楚。在这里,我们证明了亚致死过氧化氢(H2O2, 0.1 mM)暴露在人表皮黑素细胞中可诱导强大的促炎反应,而不依赖于细胞凋亡或焦亡。这种反应是由线粒体DNA (mtDNA)的选择性细胞质释放驱动的,线粒体DNA激活了干扰素基因的环GMP-AMP合成酶刺激因子(cGAS-STING)途径。在机制上,电压依赖性阴离子通道1 (VDAC1)寡聚化与线粒体通透性过渡孔(mPTP)开放协同介导mtDNA释放。VDAC1基因敲低和药物抑制均可阻断mtDNA泄漏和下游细胞因子的产生。在h2o2诱导的白癜风小鼠中,皮内给药VDAC1寡聚化抑制剂VBIT-4可恢复黑色素沉着,减少CD8+ T细胞浸润,减轻皮肤炎症。这些发现确定了VDAC1依赖性mtDNA释放是黑素细胞先天免疫激活的关键驱动因素,并突出了VDAC1作为白癜风早期干预的潜在药物治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


