Synthesis, characterization, and biological evaluation of novel xanthate ligands and their divalent metal complexes: DFT calculations and molecular docking studies
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
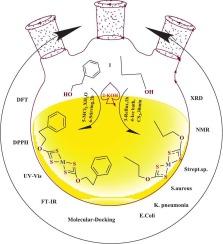
This study presents the synthesis of two novel xanthate ligands—potassium phenylethyl xanthate (L1) and potassium propyl xanthate (L2)—and their coordination complexes with Co(II), Ni(II), Cu(II), and Zn(II) ions. Comprehensive characterization—including elemental analysis, FT-IR, AAS, UV–Vis, and 1H NMR spectroscopy—confirmed the bidentate coordination mode of the ligands. Conductivity measurements indicated that the complexes are non-electrolytic, while XRD data revealed crystalline formation (particle size 16–26 nm; crystallinity index 29–68 %), and magnetic susceptibility and electronic spectra adopting tetrahedral geometries. Antibacterial screening against E. coli, K. pneumoniae, S. aureus, and Streptococcus sp. revealed negligible activity for the free ligands (zones 1.5–4 mm; MIC >100 μg/mL). Metal coordination enhanced potency, with Cu(II) complexes showing the strongest effects (zones 10.5–27 mm; MIC 6.25 μg/mL), followed by Ni(II) and Co(II), while Zn(II) derivatives were least active. Compared with ciprofloxacin (zones 20–26 mm; MIC 0.781–3.125 μg/mL), Cu(II) complexes displayed competitive antibacterial performance, indicating their promise as alternative antimicrobial agents. Antioxidant potential, assessed via the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, revealed that the Cu(II) and Ni(II) complexes achieved IC₅₀ (half-maximal inhibitory concentration) values between 55.0 and 77.4 μM, compared to 20.7 μM for ascorbic acid, indicating marked radical-scavenging ability. Molecular docking studies with human serum albumin (PDB 1H9Z) yielded binding affinities ranging from −4.0 to −9.2 kcal/mol, suggesting stable protein–ligand interactions. Density Functional Theory (DFT) investigations—addressing optimized structures, HOMO–LUMO energy gaps (3.044–4.756 eV), global reactivity parameters, and dipole moments—further substantiated the electronic stabilization conferred by metal coordination. Overall, this integrative experimental and computational study underscores the potential of these xanthate-based metal complexes as chemically stable and biologically active agents.
新型黄原酸配体及其二价金属配合物的合成、表征和生物学评价:DFT计算和分子对接研究
本文合成了两种新型黄药配体——苯乙基黄药钾(L1)和丙基黄药钾(L2),并与Co(II)、Ni(II)、Cu(II)和Zn(II)离子配合。元素分析、红外光谱、原子吸收光谱、紫外可见光谱和核磁共振氢谱等综合表征证实了配体的双齿配位模式。电导率测试表明配合物是非电解的,而XRD数据显示晶体形成(粒径16-26 nm,结晶度指数29 - 68%),磁化率和电子能谱呈四面体几何形状。对大肠杆菌、肺炎克雷伯菌、金黄色葡萄球菌和链球菌的抗菌筛选显示,游离配体的活性可以忽略不计(区域为1.5-4 mm; MIC为100 μg/mL)。金属配位增强了药效,其中Cu(II)配合物的作用最强(区域10.5 ~ 27 mm, MIC为6.25 μg/mL),其次是Ni(II)和Co(II), Zn(II)衍生物的活性最低。与环丙沙星(20 ~ 26 mm, MIC 0.781 ~ 3.125 μg/mL)相比,Cu(II)配合物具有较强的抗菌性能,有望成为新型抗菌药物。通过2,2-二苯基-1-吡啶酰肼(DPPH)自由基清除试验评估的抗氧化潜力显示,Cu(II)和Ni(II)配合物的IC₅0(半最大抑制浓度)值在55.0和77.4 μM之间,而抗坏血酸的IC₅0(半最大抑制浓度)为20.7 μM,表明具有明显的自由基清除能力。与人血清白蛋白(PDB 1H9Z)的分子对接研究发现,结合亲和度在- 4.0至- 9.2 kcal/mol之间,表明蛋白质与配体的相互作用稳定。密度泛函理论(DFT)研究——寻址优化结构、HOMO-LUMO能隙(3.044-4.756 eV)、整体反应性参数和偶极矩——进一步证实了金属配位所赋予的电子稳定性。总的来说,这项综合实验和计算研究强调了这些黄药基金属配合物作为化学稳定和生物活性剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


