Sclareol-based natural nanoparticles with adamantane moieties exert anticancer effects against non-small cell lung carcinoma cells
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Synthesis of novel derivatives of sclareol bearing an adamantane moiety was achieved aiming to improve cytotoxicity of sclareol. Novel compounds exhibited up to 100 times more pronounced effects compared to the sclareol against non-small cell lung carcinoma cell lines NCI-H460 and its multidrug resistant variant NCI-H460/R. Nine derivatives (11b, 11c, 11i, 12a, 12b, 12c, 12e, 12f, and 12 g) caused cell growth inhibition below 50 % at 1 μM concentration in NCI-H460 cells, and four derivatives (11c, 11i, 12b and 12e) showed similar activity against NCI-H460/R cells. Notably, compound 12b exhibited selectivity towards cancer cells, as well as collateral sensitivity being more potent against MDR cells. Sclareol, as well as novel derivatives 12a, 12b, 12c, 12e, 12f, and 12 g increased accumulation of rhodamine 123 which suggested their potential to modulate P-glycoprotein (P-gp) activity. Compound 12b emerged as inhibitor of P-gp and 12e and 12f as P-gp substrates. Furthermore, 12b, 12e and 12f induced reversal of doxorubicin resistance. Cell death analysis indicated necrosis as a primary type of cell death in NCI-H460 with significant increase in late apoptosis in MDR cells. We discovered for the first time that sclareol can spontaneously form negatively charged nanoparticles by being dissolved in ultrapure water. Adamantyl derivatives 12a-c and 12e-g and parental diamine derivatives 8c-f formed positively charged nanoparticles, implying that they can bind to the negatively charged cellular membrane and penetrate cancer cells. The unique nanoparticle characteristics combined with the significant cytotoxicity of the novel adamantane-sclareol derivatives underscore their potential as promising candidates for advanced anticancer nanotherapeutic applications.
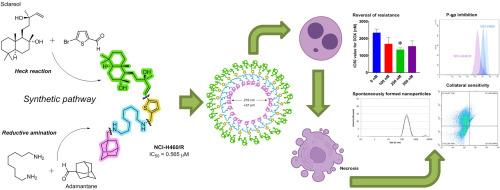
含金刚烷的天然纳米粒子对非小细胞肺癌细胞具有抗癌作用
合成了一种含有金刚烷基团的新型巩膜醇衍生物,以提高巩膜醇的细胞毒性。新化合物对非小细胞肺癌细胞株NCI-H460及其多药耐药变体NCI-H460/R的抑制作用比雌核酚高100倍。9个衍生物(11b、11c、11i、12a、12b、12c、12e、12f和12g)在1 μM浓度下对NCI-H460/R细胞的生长抑制作用低于50%,4个衍生物(11c、11i、12b和12e)对NCI-H460/R细胞的抑制作用相似。值得注意的是,化合物12b表现出对癌细胞的选择性,以及对耐多药细胞更有效的附带敏感性。sareol及其新衍生物12a、12b、12c、12e、12f和12g增加罗丹明123的积累,表明它们可能调节p -糖蛋白(P-gp)活性。化合物12b是P-gp的抑制剂,12e和12f是P-gp的底物。此外,12b、12e和12f诱导阿霉素耐药逆转。细胞死亡分析表明坏死是NCI-H460的主要细胞死亡类型,MDR细胞的晚期凋亡显著增加。我们首次发现,在超纯水中溶解后,巩膜醇可以自发形成带负电的纳米颗粒。金刚烷基衍生物12a-c和12e-g以及亲本二胺衍生物8c-f形成带正电的纳米粒子,这意味着它们可以结合带负电的细胞膜并穿透癌细胞。独特的纳米颗粒特性与新型金刚烷烷-核核醇衍生物的显著细胞毒性相结合,强调了它们作为先进抗癌纳米治疗应用的有希望的候选者的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


