Fe-doping effect on CoMoO4 for electrochemical oxygen evolution in neutral media
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
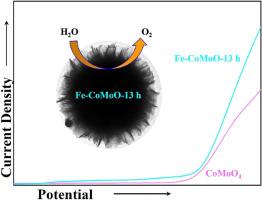
As an economically viable and environmentally benign electrocatalyst, CoMoO4 has emerged as a promising alternative to noble metal-based water oxidation catalysts in recent years. However, the oxygen evolution reaction (OER) under neutral condition presents a significantly greater challenge compared to reactions occurring in acidic or alkaline electrolytes. Therefore, to further enhance the OER activity of CoMoO4 under neutral conditions, we adopted an Fe-doping strategy. By precisely controlling the synthesis time, we successfully developed a series of Fe-CoMoO-X samples with varying morphologies (where X represents the synthesis time, ranging from 10 to 16 h) for electrocatalytic OER. By integrating multiple material characterization techniques with advanced electrochemical evaluation methods, we confirmed that the synthesis time profoundly influences the morphology of the Fe-CoMoO-X samples, which directly correlates with their catalytic performance for oxygen evolution. Moreover, all Fe-doped samples demonstrate markedly enhanced electrocatalytic activity compared to pristine CoMoO4. Notably, the Fe-CoMoO-13 h sample exhibits the most superior oxygen evolution performance, achieving an overpotential of only 380 mV at a 10 mA cm−2 (Tafel slope of 289 mV dec−1), substantially lower than that of pure CoMoO4 (400 mV at 10 mA cm−2; Tafel slope of 403 mV dec−1). This improvement can be attributed to the partial substitution of Co atoms with Fe atoms in the doped CoMoO4 structure, which modulates its electronic properties and enhances charge transfer kinetics.
fe掺杂对CoMoO4在中性介质中电化学析氧的影响
CoMoO4作为一种经济可行且环境友好的电催化剂,近年来已成为贵金属基水氧化催化剂的一种有前景的替代品。然而,与在酸性或碱性电解质中发生的反应相比,中性条件下的析氧反应(OER)具有更大的挑战性。因此,为了进一步提高CoMoO4在中性条件下的OER活性,我们采用了fe掺杂策略。通过精确控制合成时间,我们成功地开发了一系列具有不同形貌的Fe-CoMoO-X样品(其中X代表合成时间,范围从10到16小时),用于电催化OER。通过将多种材料表征技术与先进的电化学评价方法相结合,我们证实了合成时间对Fe-CoMoO-X样品的形貌有深远的影响,这与它们的析氧催化性能直接相关。此外,与原始CoMoO4相比,所有fe掺杂样品都表现出明显增强的电催化活性。值得注意的是,fe - comoo - 13h样品表现出最优越的析氧性能,在10 mA cm−2时的过电位仅为380 mV (Tafel斜率为289 mV dec−1),大大低于纯CoMoO4 (10 mA cm−2时的过电位为400 mV, Tafel斜率为403 mV dec−1)。这种改进可归因于Co原子在掺杂的CoMoO4结构中部分取代了Fe原子,从而调节了其电子性质并增强了电荷转移动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


