Design, synthesis and biological evaluation of novel quinazolinedione derivatives as non-covalent SARS-CoV-2 3CL protease inhibitors
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
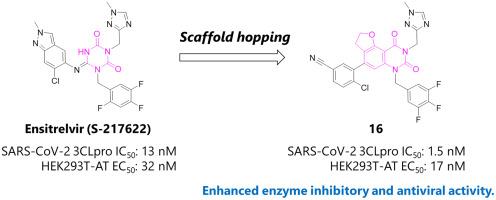
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has spread around the world since 2020, affecting many people and placing a heavy burden on medical facilities and the economy. The 3C-like protease (3CLpro) of SARS-CoV-2 is an essential enzyme for viral replication, and has been therefore attracting attention as a drug target. With the aim of creating novel non-covalent 3CLpro inhibitors, we planned a scaffold hopping transformation starting with ensitrelvir, which was discovered by Shionogi. Optimization of the substituents at the 5- and 7-positions of the newly designed quinazolinedione scaffold led to the discovery of compound 16, which exceeds ensitrelvir in enzyme inhibitory and antiviral activities. We solved the X-ray co-crystal structure of our synthesized inhibitor and 3CLpro, and clarified the interaction that contributes to its high activity. The newly discovered compound has shown good results in terms of metabolic stability and oral absorption in rat PK studies. It is expected to be a good lead compound for finding superior 3CLpro inhibitors.
新型非共价sars -cov - 23cl蛋白酶抑制剂喹唑啉二酮衍生物的设计、合成及生物学评价
自2020年以来,导致2019冠状病毒病(COVID-19)的严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)已在全球蔓延,影响了许多人,给医疗设施和经济带来了沉重负担。SARS-CoV-2的3c样蛋白酶(3CLpro)是病毒复制的必需酶,因此作为药物靶点受到关注。为了创造新的非共价3CLpro抑制剂,我们计划从盐野义发现的ensitrelvir开始进行支架跳跃转化。对新设计的喹唑啉二酮支架的5位和7位取代基进行优化,发现了化合物16,其酶抑制和抗病毒活性超过ensitrelvir。我们解决了我们合成的抑制剂和3CLpro的x射线共晶结构,并澄清了导致其高活性的相互作用。新发现的化合物在大鼠PK研究中显示出良好的代谢稳定性和口服吸收效果。它有望成为寻找优质3CLpro抑制剂的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


