Impact of surgical interventions on postoperative cognitive dysfunction: A focus on gene expression in the hippocampus and cerebral cortex and peripheral blood cells
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Background
Postoperative cognitive dysfunction (POCD) is a common surgical complication with unclear mechanisms. This study aimed to characterize hippocampal, cortical, and PBMC gene expression changes after surgery to identify biomarkers and therapeutic targets.
Methods
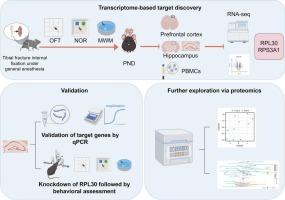
Male C57BL/6 mice underwent tibial fracture with intramedullary fixation. Behavioral tests (MWM, OFT, NOR) before and after surgery classified animals into control, surgery without POCD (G1), and surgery with POCD (G2) using Z-scores. Bulk RNA-seq was performed on hippocampus, cortex, and PBMCs, followed by network and pathway analyses. qRT-PCR validated hub genes, including RPL30 and RPS3A1. Functional assays involved hippocampal RPL30 knockdown in POCD mice with behavioral assessment. Hippocampal proteomics was performed and integrated with transcriptomic data to link gene expression with protein function and metabolic alterations.
Results
The study identified DEGs involved in cortical protein export, nicotine metabolism, and mitophagy; hippocampal endocannabinoid and IL-17 signaling; and PBMC lysosomal and MAPK pathways. Twelve hub genes, including RPL30 and RPS3A1, were identified; qRT-PCR confirmed their upregulation in POCD. RPL30 knockdown improved cognition. Proteomics revealed 80 differentially expressed proteins, with pathways related to TORC1 signaling, histone modification, lysosomal regulation, and energy metabolism.
Conclusions
This study identified key genes, proteins, and molecular pathways associated with postoperative cognitive dysfunction, including RPL30, RPS3A1, and pathways related to TORC1 signaling, histone modification, lysosomal regulation, and energy metabolism. These findings provide novel biomarkers and potential therapeutic targets for the early detection and intervention of POCD.
手术干预对术后认知功能障碍的影响:海马体、大脑皮层和外周血细胞的基因表达
背景术后认知功能障碍(POCD)是一种常见的手术并发症,机制尚不清楚。本研究旨在描述手术后海马、皮质和PBMC基因表达的变化,以确定生物标志物和治疗靶点。方法采用C57BL/6小白鼠胫骨骨折髓内固定。术前和术后行为测试(MWM, OFT, NOR)将动物分为对照组、无POCD手术组(G1)和有POCD手术组(G2)。在海马、皮质和pbmc上进行大量rna测序,然后进行网络和通路分析。qRT-PCR验证了枢纽基因,包括RPL30和RPS3A1。功能分析涉及POCD小鼠海马RPL30敲除行为评估。海马蛋白质组学研究与转录组学数据相结合,将基因表达与蛋白质功能和代谢改变联系起来。结果发现deg参与皮层蛋白输出、尼古丁代谢和线粒体自噬;海马内源性大麻素和IL-17信号;以及PBMC溶酶体和MAPK途径。共鉴定到12个中心基因,包括RPL30和RPS3A1;qRT-PCR证实了它们在POCD中的上调。RPL30敲除可改善认知。蛋白质组学揭示了80个差异表达蛋白,其途径与TORC1信号传导、组蛋白修饰、溶酶体调节和能量代谢有关。本研究确定了与术后认知功能障碍相关的关键基因、蛋白和分子通路,包括RPL30、RPS3A1,以及与TORC1信号传导、组蛋白修饰、溶酶体调控和能量代谢相关的通路。这些发现为POCD的早期检测和干预提供了新的生物标志物和潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


