CO2 reduction catalysis of CO doped Cu nanoparticles anchored to graphene: A DFT study
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study investigates the structural and electronic properties of graphene-supported Cu19 and Cu18Co nanocluster catalysts using density functional theory (DFT). The stable configurations of the catalysts were optimized, and their corresponding structural features and charge distribution characteristics were analyzed. Furthermore, we systematically explored the possible reaction mechanisms for CO2 reduction to C1 products on both Cu18Co/G and Cu19/G catalysts. Through comparative analysis of reaction pathway activation energies, the optimal routes for CO2 catalytic reduction to four distinct C1 products were identified. The study reveals that for CO production, the rate-determining step activation energies are 1.48 eV on Cu18Co/G and 0.68 eV on Cu19/G, respectively. For HCOOH formation, the corresponding activation barriers are 1.31 eV and 1.09 eV for Cu18Co/G and Cu19/G catalysts. Meanwhile, the activation energies of the rate-determining steps for the optimal pathways of CO2 reduction to CH3OH were determined to be 1.88 eV and 1.24 eV for Cu18Co/G and Cu19/G, respectively. These results indicate that the pure copper nanocluster catalyst Cu19/G exhibits superior activity for the catalytic reduction of CO2 to three major products: CO, HCOOH and CH3OH. In contrast, for CH4 formation, the activation barriers of the rate-determining steps were calculated to be 1.48 eV and 1.60 eV on Cu18Co/G and Cu19/G catalysts. Our findings reveal that the Co-doped Cu18Co/G catalyst promotes selective CH4 production in CO2 reduction. Our systematic evaluation reveals distinct product selectivity trends for CO2 reduction to C1 products between the two catalysts. The Cu19/G system exhibits the following activity hierarchy: CO > HCOOH > CH3OH > CH4, indicating unfavorable CH4 formation kinetics. The Cu18Co/G catalyst exhibits a distinct product selectivity trend for CO2 reduction to C1 products: HCOOH > CH4 = CO > CH3OH. The CH3OH yield was found to be the lowest among all products. Our findings aim to provide theoretical guidance for the design and optimization of CO2 catalysts.
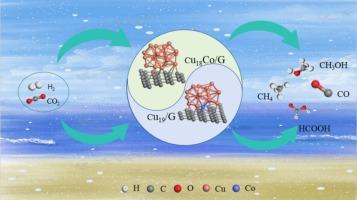
锚定在石墨烯上的CO掺杂Cu纳米颗粒的CO2还原催化:DFT研究
利用密度泛函理论(DFT)研究了石墨烯负载Cu19和Cu18Co纳米团簇催化剂的结构和电子性能。优化了催化剂的稳定构型,分析了催化剂的结构特征和电荷分布特征。此外,我们系统地探索了Cu18Co/G和Cu19/G催化剂上CO2还原为C1产物的可能反应机理。通过对反应途径活化能的比较分析,确定了CO2催化还原生成四种不同C1产物的最佳途径。研究表明,对于CO的生成,Cu18Co/G和Cu19/G上的速率决定步骤活化能分别为1.48 eV和0.68 eV。对于生成HCOOH, Cu18Co/G和Cu19/G催化剂对应的激活势垒分别为1.31 eV和1.09 eV。同时,Cu18Co/G和Cu19/G的CO2还原为CH3OH的最佳途径的速率决定步骤的活化能分别为1.88 eV和1.24 eV。这些结果表明,纯铜纳米簇催化剂Cu19/G对CO、HCOOH和CH3OH三种主要产物的催化还原表现出优异的活性。而在Cu18Co/G和Cu19/G催化剂上,CH4生成的速率决定步骤的激活势垒分别为1.48 eV和1.60 eV。我们的研究结果表明,共掺杂Cu18Co/G催化剂促进了CO2还原过程中选择性产生CH4。我们的系统评价揭示了两种催化剂之间CO2还原为C1产物的明显的产物选择性趋势。Cu19/G体系表现出以下活性等级:CO >; HCOOH > CH3OH > CH4,表明不利的CH4形成动力学。Cu18Co/G催化剂对CO2还原为C1产物表现出明显的选择性趋势:HCOOH >; CH4 = CO > CH3OH。CH3OH产率是所有产物中最低的。本研究旨在为CO2催化剂的设计和优化提供理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


