Predicting inclusion free energy for clomiphene in HPβCD cyclodextrin using QC/MD approach
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Low aqueous solubility has motivated the development of new formulations through complexation with β-cyclodextrins (βCD). In this study, the inclusion of clomiphene isomers (ECL and ZCL) into 2-hydroxypropyl-βCD (HPβCD) was investigated using a sequential approach combining molecular dynamics (MD) simulations for structural sampling with quantum computational (QC) calculations for free energy estimation. The experimental association constant and inclusion free energy (3838 M−1; −4.836 kcal mol−1) were satisfactorily reproduced by a weighted average over 2000 configurations (4267 M−1; −4.90 kcal mol−1). Beyond providing mechanistic insights into the inclusion process, this work extends the applicability of the QC/MD methodology to flexible systems with high association constants and examines the effect of two force fields (GAFF2 and GLYCAM06) on configurational sampling. The results highlight the importance of MD sampling prior to QC calculations, as docking followed by QC alone fails to yield reliable association constants.
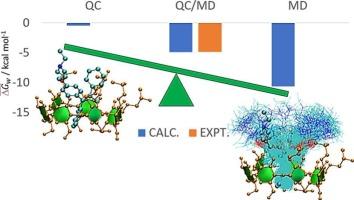
用QC/MD法预测克罗米芬在HPβCD环糊精中的包合自由能
低水溶性促使了与β-环糊精(βCD)络合的新配方的发展。本研究采用分子动力学(MD)模拟结构采样和量子计算(QC)计算自由能相结合的顺序方法研究了氯米芬异构体(ECL和ZCL)在2-羟丙基-βCD (HPβCD)中的包合。通过对2000种构型(4267 M−1;- 4.90 kcal mol−1)的加权平均,实验缔合常数和包合自由能(3838 M−1;- 4.836 kcal mol−1)得到了满意的结果。除了提供包含过程的机理见解之外,这项工作还将QC/MD方法的适用性扩展到具有高关联常数的柔性系统,并研究了两个力场(GAFF2和GLYCAM06)对构型采样的影响。结果强调了在QC计算之前进行MD采样的重要性,因为单独的QC对接不能产生可靠的关联常数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


