Fragment-based drug discovery: A graphical review
Q2 Agricultural and Biological Sciences
Current Research in Pharmacology and Drug Discovery
Pub Date : 2025-01-01
DOI:10.1016/j.crphar.2025.100233
引用次数: 0
Abstract
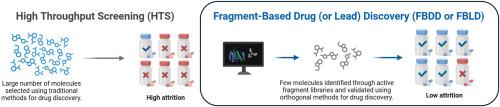
Three decades after its introduction, fragment-based drug (or lead) discovery (FBDD or FBLD) has become a mature and powerful strategy for generating novel leads, offering distinct advantages for challenging or previously “undruggable” targets where traditional screening (e.g., high throughput screening) often fails. The FBDD approach identifies low molecular weight fragments (MW < 300 Da) that bind weakly to a target; these interactions are detected using highly sensitive biophysical methods such as NMR, X-ray crystallography, and SPR. These initial hits are then optimised into potent leads through structure-guided strategies, including fragment growing, linking, or merging. This graphical review illustrates the modern FBDD workflow, highlighting the critical integration of experimental and computational methods. We discuss how innovations in library design, hybrid screening platforms, and the application of AI/ML are accelerating discovery cycles and improving hit validation. The power of this approach is demonstrated through case studies of FDA-approved drugs, including Vemurafenib and Venetoclax, which progressed from simple fragments to transformative medicines. Finally, we provide an outlook on the future of FBDD as it continues to evolve with emerging technologies to push the boundaries of drug discovery.
基于片段的药物发现:图形回顾
在引入三十年后,基于片段的药物(或先导物)发现(FBDD或FBLD)已成为产生新型先导物的成熟而强大的策略,为具有挑战性或以前“不可药物”的靶标提供了独特的优势,而传统筛选(例如高通量筛选)往往失败。FBDD方法识别与靶标结合较弱的低分子量片段(MW < 300 Da);这些相互作用是使用高灵敏度的生物物理方法,如核磁共振、x射线晶体学和SPR来检测的。然后通过结构导向策略(包括片段增长、链接或合并)将这些初始命中优化为有效的线索。这张图表说明了现代FBDD工作流程,突出了实验和计算方法的关键集成。我们讨论了图书馆设计、混合筛选平台和AI/ML应用的创新如何加速发现周期和改进命中验证。这种方法的力量通过fda批准的药物的案例研究得到了证明,包括Vemurafenib和Venetoclax,这些药物从简单的片段发展到变革性药物。最后,我们展望了FBDD的未来,因为它将随着新兴技术的发展而不断发展,以推动药物发现的界限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Pharmacology and Drug Discovery
Agricultural and Biological Sciences-Animal Science and Zoology
CiteScore
6.40
自引率
0.00%
发文量
65
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


