Circadian regulation of vascular function: Metabolism as a link from molecular mechanisms to clinical implications
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-09
DOI:10.1016/j.bbadis.2025.168048
引用次数: 0
Abstract
Circadian rhythms act as central coordinators of vascular physiology, synchronizing metabolic and hemodynamic processes across different vascular beds. Cell-autonomous clocks dynamically regulate vascular functions, including vasodilation and inflammatory responses, in endothelial cells, smooth muscle cells, and fibroblasts. Emerging evidence indicates extensive crosstalk with metabolic cell death pathways, particularly lipophagy-mediated lipid turnover, redox stress-triggered disulfidptosis, and glucotoxicity-induced metabolic collapse, all of which display circadian rhythmicity. Disruption of these pathways, such as in shift workers or individuals carrying clock gene mutation, significantly increases the risk of hypertension, atherosclerosis, and microvascular dysfunction. In this review, we highlight translational strategies that leverage circadian biology, including chronotherapy, pharmacological modulation of core circadian clock components, light-dark synchronization, and lifestyle interventions. We also emphasize that future research should aim to decode the spatiotemporal regulation of circadian-metabolic networks, which may offer novel insights for precision medicine approaches targeting vascular metabolic disorders.
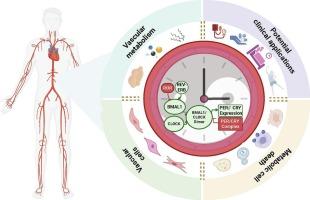
血管功能的昼夜节律调节:从分子机制到临床意义的代谢联系。
昼夜节律作为血管生理学的中心协调者,同步不同血管床的代谢和血流动力学过程。细胞自主时钟动态调节血管功能,包括内皮细胞、平滑肌细胞和成纤维细胞的血管舒张和炎症反应。新出现的证据表明,代谢细胞死亡途径之间存在广泛的串扰,尤其是脂质吞噬介导的脂质转换、氧化还原应激引发的二亢下垂和糖毒性诱导的代谢崩溃,所有这些都表现出昼夜节律性。这些通路的破坏,如轮班工人或携带时钟基因突变的个体,显著增加高血压、动脉粥样硬化和微血管功能障碍的风险。在这篇综述中,我们重点介绍了利用昼夜节律生物学的翻译策略,包括时间疗法、核心生物钟成分的药理调节、明暗同步和生活方式干预。我们还强调,未来的研究应致力于解码昼夜代谢网络的时空调节,这可能为针对血管代谢疾病的精准医学方法提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


