Biliverdin alleviates cerebral ischemia-reperfusion injury by inhibiting autophagy via the lncRNA TUG1 /miR-204-5p/P4HB axis
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Background
Biliverdin (BV), a heme metabolite, alleviates cerebral ischemia-reperfusion injury (CIRI) by regulating non-coding RNAs. Long non-coding RNA taurine-upregulated gene 1 (lncRNA TUG1) has been implicated in CIRI pathogenesis. This study investigates the mechanism of lncRNA TUG1 in BV-mediated CIRI mitigation in vivo and in vitro.
Methods
In vivo CIRI was induced in rats via middle cerebral artery occlusion-reperfusion (MCAO/R), while in vitro CIRI was modeled in PC12 cells using oxygen-glucose deprivation/reoxygenation (OGD/R). BV was administered prior to reperfusion (in vivo) or reoxygenation (in vitro). Western blot and transmission electron microscopy (TEM) were used to evaluate BV's neuroprotective effects and its impact on autophagy. Quantitative real-time PCR (qPCR) and dual-luciferase reporter assays were performed to assess the expression levels of lncRNA TUG1, miR-204-5p, and P4HB mRNA, and their interactions. Functional rescue experiments were conducted by plasmid/siRNA transfection to overexpress lncRNA TUG1 or knockdown miR-204-5p. Autophagy and apoptosis were evaluated using MDC staining, Western blot, and flow cytometry.
Results
BV significantly reduced neurological deficit scores and neuronal apoptosis in CIRI rats, decreased the LC3 II/I ratio, Beclin1, and P4HB protein levels, and reversed rapamycin-induced autophagy activation. BV downregulated lncRNA TUG1 and P4HB mRNA while upregulating miR-204-5p. Overexpression of lncRNA TUG1 or inhibition of miR-204-5p exacerbated OGD/R-induced cell damage through enhanced autophagy, thereby abolishing BV's protective effects.
Conclusion
BV alleviates CIRI by suppressing excessive autophagy via the lncRNA TUG1/miR-204-5p/P4HB axis.
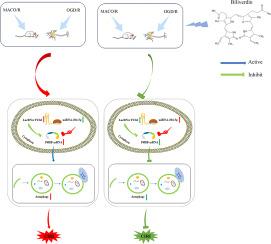
胆绿素通过lncRNA TUG1 /miR-204-5p/P4HB轴抑制自噬,减轻脑缺血再灌注损伤。
背景:胆绿素(BV)是一种血红素代谢物,通过调节非编码rna减轻脑缺血再灌注损伤(CIRI)。长链非编码RNA牛磺酸上调基因1 (lncRNA TUG1)参与了CIRI的发病机制。本研究在体内和体外研究lncRNA TUG1在bv介导的CIRI缓解中的机制。方法:采用大脑中动脉闭塞再灌注法(MCAO/R)在体内诱导大鼠CIRI,采用氧-葡萄糖剥夺/再氧化法(OGD/R)在PC12细胞中模拟体外CIRI。BV在再灌注(体内)或再氧合(体外)之前给予。采用Western blot和透射电镜(TEM)观察BV的神经保护作用及其对自噬的影响。采用实时荧光定量PCR (qPCR)和双荧光素酶报告基因检测来评估lncRNA TUG1、miR-204-5p和P4HB mRNA的表达水平及其相互作用。通过质粒/siRNA转染,过表达lncRNA TUG1或敲低miR-204-5p,进行功能挽救实验。采用MDC染色、Western blot和流式细胞术观察细胞自噬和凋亡情况。结果:BV显著降低CIRI大鼠神经功能缺损评分和神经元凋亡,降低LC3 II/I比值、Beclin1和P4HB蛋白水平,逆转雷帕霉素诱导的自噬激活。BV下调lncRNA TUG1和P4HB mRNA,上调miR-204-5p。过表达lncRNA TUG1或抑制miR-204-5p会通过增强自噬加剧OGD/ r诱导的细胞损伤,从而消除BV的保护作用。结论:BV通过lncRNA TUG1/miR-204-5p/P4HB轴抑制过度自噬,从而缓解CIRI。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


