CRABS-ROC, A respirometry protocol for overcoming substrate limitations, reveals excess brain mitochondrial Complex I capacity
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Mitochondrial bioenergetic competency in cells is frequently assessed by the Mito Stress Test protocol, which includes uncoupler addition for evaluating respiratory capacity. The uncoupled oxygen consumption rate (OCR) is usually defined as maximal respiration, with little consideration of whether the measured rate is restricted by substrate supply. In this study, we show that the uncoupled OCR is substrate-limited in rat primary cortical neurons and isolated mouse forebrain synaptosomes. We use a different respirometry protocol we name CRABS-ROC (Complex Respirometry Assay Bypassing Substrate-Restricted Oxygen Consumption) that enables evaluation of individual electron transport chain (ETC) complex capacities using saturating levels of substrates to bypass this restriction. Optimization of the cytochrome c concentration was critical for ETC complex capacity assessment. Applying CRABS-ROC to primary cortical neurons reveals >2-fold excess Complex I capacity beyond the uncoupled OCR of cells metabolizing glucose and pyruvate. Furthermore, we demonstrate that CRABS-ROC can expose a Complex I deficit in isolated harlequin mutant brain mitochondria that display wild-type levels of Complex I-substrate-linked respiration despite having about half the normal level of Complex I. Thus, CRABS-ROC should be broadly useful for studies on mitochondrial function because it can both reveal excess ETC capacity and unmask ETC alterations that may be missed by the most widely used methods.
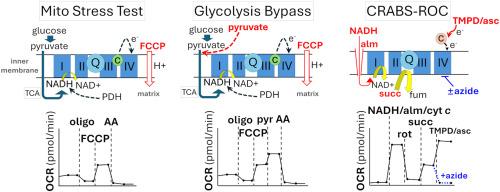
螃蟹- roc,一种克服底物限制的呼吸测量方案,揭示了过量的脑线粒体复合体I容量。
细胞中的线粒体生物能量能力经常通过水户应激测试方案进行评估,其中包括用于评估呼吸能力的解耦器添加。解耦耗氧速率(OCR)通常被定义为最大呼吸,很少考虑所测速率是否受底物供应的限制。在这项研究中,我们发现解耦OCR在大鼠初级皮质神经元和分离的小鼠前脑突触体中受到底物限制。我们使用了一种不同的呼吸测定方案,我们将其命名为crab - roc(绕过底物限制耗氧量的复杂呼吸测定法),该方案可以使用底物的饱和水平来绕过这一限制,从而评估单个电子传递链(ETC)的复杂能力。细胞色素c浓度的优化是ETC复合物容量评价的关键。将CRABS-ROC应用于初级皮质神经元显示,在代谢葡萄糖和丙酮酸的细胞的未偶联OCR之外,复合物I的容量超过了2倍。此外,我们证明了CRABS-ROC可以揭示分离的小丑突变体脑线粒体中的Complex I缺陷,这些线粒体显示出野生型的Complex I底物相关呼吸水平,尽管其Complex I水平约为正常水平的一半。因此,CRABS-ROC应该广泛用于线粒体功能的研究,因为它既可以揭示过剩的ETC能力,也可以揭示最广泛使用的方法可能忽略的ETC改变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


