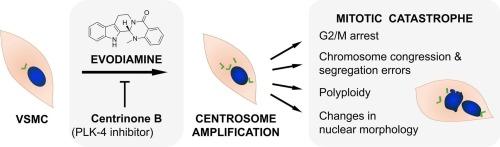
Evodiamine induces centrosome amplification with subsequent G2/M cell cycle arrest in primary vascular smooth muscle cells
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Evodiamine (EVO) is a natural product found in Tetradium ruticarpum. It inhibits vascular smooth muscle cell (VSMC) proliferation, a key mechanism in the pathogenesis of atherosclerosis and restenosis. This study characterizes the mechanism of action behind the antiproliferative activity of evodiamine in platelet derived growth factor (PDGF)-activated VSMC.
We confirmed the antiproliferative activity of EVO (0.3 and 1 µmol/L) in cultured primary VSMC by resazurin conversion and bromo-deoxyuridine (BrdU) incorporation assays, respectively, and its ability to arrest VSMC in G2/M by flow cytometric cell cycle analysis. Annexin V- Fluorescein Isothiocyanate (FITC)/propidium iodide (PI) staining and western blot analysis of caspase-3 cleavage detected low levels of apoptosis in response to 3 µmol/L EVO. We demonstrate that EVO (3 µmol/L) induces mitotic catastrophe (MC), as evidenced by characteristic nuclear morphology observed by confocal microscopy and polyploidy detected by flow cytometric DNA content analysis.
Mechanistically, we rule out DNA damage as a cause of MC by western blot analysis of phospho-Ser139 histone H2A.X (γH2A.X). Instead, EVO induces centrosome amplification involving polo-like kinase 4 (PLK4) signaling. This is evident in cells co-treated with EVO (3 µmol/L) and the PLK4 inhibitor centrinone B (CENB) at 125 nmol/L by blunted centrosome amplification and cell cycle arrest. The study concludes with a proteomic analysis of purified centrosomes, which identifies candidates involved in this mechanism.
In conclusion, evodiamine induces mitotic catastrophe via centrosome amplification in VSMC, positioning it as an antiproliferative agent with a distinct mechanism.
evolodiamine诱导原代血管平滑肌细胞中心体扩增,随后G2/M细胞周期阻滞。
Evodiamine (EVO)是一种天然产物,发现于四合金中。它抑制血管平滑肌细胞(VSMC)的增殖,这是动脉粥样硬化和再狭窄发病的关键机制。本研究探讨了evoldiine在血小板衍生生长因子(PDGF)激活的VSMC中抗增殖活性的作用机制。通过reazurin转化和溴脱氧尿苷(BrdU)结合实验,我们分别证实了EVO(0.3和1 µmol/L)在培养的原代VSMC中的抗增殖活性,并通过流式细胞术细胞周期分析证实了EVO在G2/M中抑制VSMC的能力。Annexin V-异硫氰酸荧光素(FITC)/碘化丙啶(PI)染色和caspase-3裂解的western blot分析检测到3 µmol/L EVO引起的低水平凋亡。我们证明EVO(3 µmol/L)诱导有丝分裂突变(MC),这可以通过共聚焦显微镜观察到的核形态特征和流式细胞术DNA含量分析检测到的多倍体来证明。从机制上讲,我们通过对phospho-Ser139组蛋白H2A进行western blot分析,排除了DNA损伤是MC的原因。X(γH2A.X)。相反,EVO诱导涉及polo样激酶4 (PLK4)信号的中心体扩增。这在用EVO(3 µmol/L)和PLK4抑制剂centrinone B (CENB)(125 nmol/L)共处理的细胞中很明显,通过钝化中心体扩增和细胞周期阻滞。该研究以纯化中心体的蛋白质组学分析结束,该分析确定了参与该机制的候选中心体。综上所述,evoldiine通过中心体扩增在VSMC中诱导有丝分裂突变,是一种具有独特机制的抗增殖药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


