CD300f enables microglial damage sensing, efferocytosis, and apoptotic cell metabolization after brain injury
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Microglia, the resident phagocytes of the central nervous system (CNS), continuously survey the parenchyma and its borders, acting as first responders to brain injury. Their ability to detect and react to environmental changes is mediated by a repertoire of surface receptors collectively known as the microglial sensome.
Here, we identify the lipid-sensing immunoreceptor CD300f as a key regulator of microglial responses to tissue damage and apoptotic cells. Using intravital two-photon microscopy, we show that CD300f−/− microglia fail to extend processes toward a laser-induced cortical lesion, indicating impaired detection of damage-associated cues.
In models of mild traumatic brain injury (mTBI) and intracortical injection of apoptotic cells, CD300f deficiency led to reduced recognition and clearance of dying cells resulting in the accumulation of cellular debris within the parenchyma. At later stages, apoptotic remnants were retained within CD300f−/− microglia in vivo and bone marrow–derived macrophages in vitro, suggesting defective intracellular degradation.
Proteomic analysis after a controlled cortical injury (CCI) contusion model revealed widespread dysregulation of autophagy-related and metabolic pathways, consistent with impaired efferocytosis and phagolysosomal processing. In parallel, we observed upregulation of the UDP-degrading ectonucleotidase ENTPD6 protein and downregulation of the microglial purinergic receptor P2ry6 mRNA, indicating a dysfunctional UDP–P2RY6 axis that may underlie impaired damage sensing and phagocytic initiation.
Despite greater histological preservation, CD300f−/− mice exhibited worse long-term functional recovery after brain injury.
Together, these findings highlight CD300f as a key damage-associated molecular pattern (DAMP) receptor that integrates purinergic signaling, efferocytosis, and metabolic adaptation, highlighting its essential role in coordinating microglial responses to CNS injury.
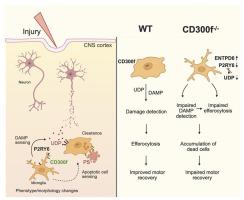
CD300f促进脑损伤后小胶质细胞损伤感知、efferocytosis和凋亡细胞代谢。
小胶质细胞是中枢神经系统(CNS)的常驻吞噬细胞,它不断地检查脑实质及其边界,作为脑损伤的第一反应者。它们对环境变化的检测和反应能力是由一系列表面受体介导的,这些受体统称为小胶质感觉体。在这里,我们发现脂质敏感免疫受体CD300f是小胶质细胞对组织损伤和细胞凋亡反应的关键调节因子。使用活体双光子显微镜,我们发现CD300f-/-小胶质细胞不能将过程扩展到激光诱导的皮质病变,表明损伤相关信号的检测受损。在轻度创伤性脑损伤(mTBI)和皮质内注射凋亡细胞的模型中,CD300f缺乏导致对死亡细胞的识别和清除减少,导致细胞碎片在实质内积累。在后期,体内CD300f-/-小胶质细胞和体外骨髓源性巨噬细胞中保留了凋亡残留物,表明细胞内降解存在缺陷。控制性皮质损伤(CCI)挫伤模型后的蛋白质组学分析显示,自噬相关和代谢途径普遍失调,与efferocytosis和吞噬溶酶体加工受损一致。同时,我们观察到udp降解外核苷酸酶ENTPD6蛋白的上调和小胶质嘌呤能受体P2ry6 mRNA的下调,表明UDP-P2RY6轴功能失调可能是损伤感知和吞噬起始受损的基础。尽管CD300f-/-小鼠在脑损伤后表现出更差的长期功能恢复。总之,这些发现将CD300f定位为一种关键的损伤相关分子模式(DAMP)受体,它整合了嘌呤能信号、efferocytosis和代谢适应,突出了其在协调小胶质细胞对中枢神经系统损伤的反应中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


