Redox-dependent pathways in the pro-thermogenic effects of cysteine restriction
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
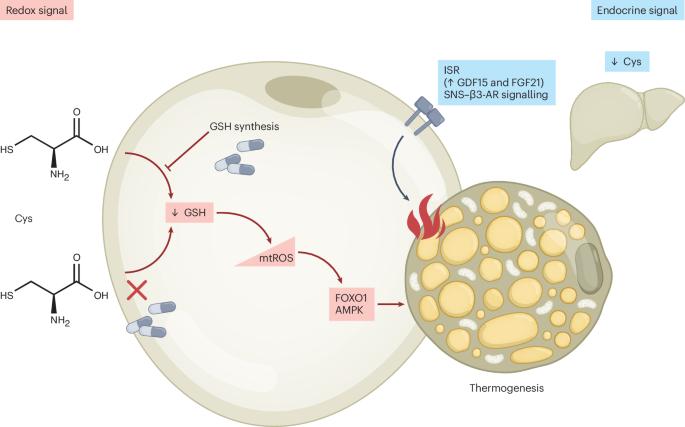
半胱氨酸限制的促热作用中的氧化还原依赖途径
在他们最近的文章中,Lee等人1优雅地证明了饮食半胱氨酸限制通过独立于UCP1的交感神经系统途径诱导快速体重减轻和脂肪组织重塑。与此同时,Varghese等人报告说,同样的饮食限制点燃了一个强大的产热程序,脂肪量的减少和综合应激反应的激活。值得注意的是,在GDF15和FGF21敲除的小鼠中,循环GDF15和FGF21放大了表型,但不是必不可少的。肝脏和脂肪组织中含有半胱氨酸的抗氧化剂谷胱甘肽(GSH)的显著下降强调了氧化还原失衡是一个早期事件。总之,这两项研究强调了半胱氨酸限制作为减肥干预的翻译潜力,但对脂肪细胞内将半胱氨酸可用性与产热输出相结合的氧化还原机制留下了关键问题。GSH的消耗已被证明可以减少肥胖,足以改善胰岛素敏感性,并诱导脂肪细胞和体内的产热3,4,5。我们的实验室证明,用丁硫氨酸亚砜抑制γ-谷氨酰半胱氨酸连接酶可降低GSH,提高线粒体活性氧(mtROS),激活氧化还原敏感转录因子FOXO1,从而诱导脂肪细胞中的Ucp1和Ppargc1a。在培养的脂肪细胞中触发的基因程序反映了体内的反应,表明脂肪细胞本身是独立于系统输入的足够的氧化还原传感器。补充半胱氨酸可以逆转这些影响,认为脂肪组织的局部氧化还原状态是产热激活的守门人。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


