EMC2 promotes triple negative breast cancer growth by protecting FDFT1 from endoplasmic reticulum associated degradation to impair ferroptosis susceptibility
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cholesterol biosynthesis is more activated in triple negative breast cancer (TNBC) than in other subtype breast cancer and plays essential role in facilitating TNBC. However, the regulatory network and how cholesterol biosynthesis contribute to TNBC development and progression are not well elucidated. Here, we found that reticulum membrane protein complex 2 (EMC2) is highly expressed in TNBC and predicts short survival of patients. In vitro and in vivo experiments displayed that EMC2 could promote TNBC growth. We also displayed that EMC2 could increase intracellular cholesterol biosynthesis by regulating Farnesyltransferase 1 (FDFT1) expression. Mechanistically, we validated that EMC2 interacted with heat shock protein 90(HSP90) to sustain FDFT1 protein quality and correctly located in the ER membrane through protecting it from endoplasmic reticulum associated degradation (ERAD). Furthermore, EMC2 decreased TNBC cell ferroptosis susceptibility through elevating intracellular cholesterol contents. Collectively, our findings shed a new insight that EMC2 is critical for boosting cholesterol biosynthesis and ferroptosis resistance. Targeting EMC2 could be a promising novel therapeutic target for TNBC treatment.
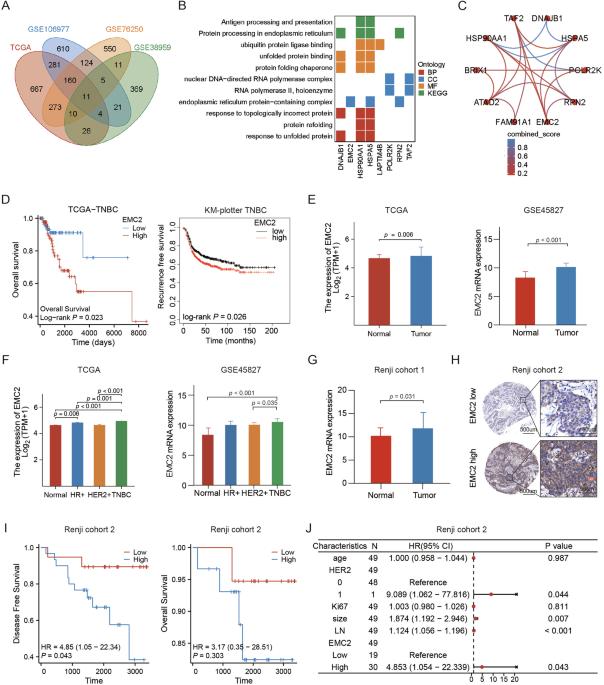
EMC2通过保护FDFT1免受内质网相关降解而损害铁下垂易感性,从而促进三阴性乳腺癌的生长。
胆固醇生物合成在三阴性乳腺癌(TNBC)中比在其他亚型乳腺癌中更活跃,并在TNBC的促进中起重要作用。然而,调控网络和胆固醇生物合成如何促进TNBC的发生和进展尚未得到很好的阐明。在这里,我们发现网状膜蛋白复合物2 (EMC2)在TNBC中高表达,并预测患者的短生存期。体外和体内实验表明,EMC2可以促进TNBC的生长。我们还发现,EMC2可以通过调节法尼基转移酶1 (FDFT1)的表达来增加细胞内胆固醇的生物合成。在机制上,我们证实了EMC2与热休克蛋白90(HSP90)相互作用,以维持FDFT1蛋白的质量,并通过保护其免受内质网相关降解(ERAD)而正确定位于内质网膜。此外,EMC2通过提高细胞内胆固醇含量降低TNBC细胞铁下垂的易感性。总的来说,我们的研究结果揭示了一个新的见解,即EMC2对促进胆固醇生物合成和铁下沉抗性至关重要。靶向EMC2可能是TNBC治疗的一个有前景的新靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


