Progressive lifespan modifications in the corpus callosum following a single concussion in juvenile male mice monitored by diffusion MRI
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Introduction
The vulnerability of white matter (WM) in acute and chronic moderate-severe traumatic brain injury (TBI) has been established. In concussion syndromes, including preclinical rodent models, lacking are comprehensive longitudinal studies spanning the mouse lifespan. We previously reported early WM modifications using clinically relevant neuroimaging and histological measures in a model of juvenile concussion at one month post injury (mpi) who then exhibited cognitive deficits at 12mpi. For the first time, we assess corpus callosum (CC) integrity across the lifespan after a single juvenile concussion utilizing diffusion MRI (dMRI).
Methods
C57Bl/6 mice were exposed to sham or two severities of closed-head concussion (Grade 1, G1, speed 2 m/s, depth 1 mm; Grade 2, G2, 3 m/s, 3 mm) using an electromagnetic impactor at postnatal day 17. In vivo diffusion tensor imaging was conducted at 1, 3, 6, 12 and 18mpi and processed for dMRI parametric maps: fractional anisotropy (FA), axial (AxD), radial (RD) and mean diffusivity (MD). Hemispheric CC and regional CC data were extracted. To identify the biological basis of altered dMRI metrics, astrocyte and microglia in the CC were characterized at 1, 12 and 18 mpi by immunohistochemistry.
Results
Hemispheric CC analysis revealed altered FA and RD trajectories following juvenile concussion. Shams exhibited a temporally linear increase in FA with age while G1/G2 mice had plateaued FA values. G2 concussed mice exhibited high variance of dMRI metrics at 18mpi, which was attributed to the heterogeneity of TBI on the anterior CC. Regional analysis of dMRI metrics at the impact site unveiled significant differences between G2 and sham mice. The dMRI findings appear to be driven, in part, by loss of astrocyte morphology.
Conclusion
For the first time, we demonstrate progressive perturbations to WM of male mice after a single juvenile concussion across their lifespan. The CC alterations were dependent on concussion severity with elevated sensitivity in the anterior CC that was related to astrocyte and microglial morphology changes. Our findings suggest that long-term monitoring of children with juvenile concussive episodes using dMRI is warranted, focusing on vulnerable WM tracts.
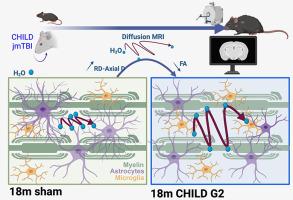
扩散MRI监测幼年雄性小鼠单次脑震荡后胼胝体的进行性寿命改变。
急慢性中重度创伤性脑损伤(TBI)中白质(WM)的易损性已被证实。在脑震荡综合征中,包括临床前啮齿动物模型,缺乏跨越小鼠寿命的全面纵向研究。我们之前报道了一个损伤后1个月(mpi)的青少年脑震荡模型的早期WM改变,该模型在12mpi时表现出认知缺陷。这是我们第一次利用扩散MRI (dMRI)评估单一青少年脑震荡后整个生命周期的胼胝体(CC)完整性。方法:C57Bl/6小鼠在出生后第17天用电磁冲击器进行假或两种严重程度的封闭式头部震荡(1级G1,速度2 m/s,深度1 mm; 2级G2, 3 m/s, 3 mm)。在1,3,6,12和18mpi下进行体内扩散张量成像,并处理dMRI参数图:分数各向异性(FA),轴向(AxD),径向(RD)和平均扩散率(MD)。提取半球CC和区域CC数据。为了确定dMRI指标改变的生物学基础,在1,12和18 mpi时,通过免疫组织化学对CC中的星形胶质细胞和小胶质细胞进行了表征。结果:半球CC分析显示青少年脑震荡后FA和RD轨迹改变。sham小鼠的FA随年龄的增长呈暂时线性增长,而G1/G2小鼠的FA值趋于稳定。G2脑震荡小鼠在18mpi时的dMRI指标表现出很高的差异,这是由于前CC的TBI异质性造成的。在冲击部位的dMRI指标的区域分析显示G2和假手术小鼠之间存在显著差异。dMRI的发现似乎部分是由星形细胞形态的丧失所驱动的。结论:我们首次证明了雄性小鼠一生中在一次幼年脑震荡后WM的渐进式扰动。CC的改变依赖于脑震荡的严重程度,前CC的敏感性升高与星形胶质细胞和小胶质细胞形态的改变有关。我们的研究结果表明,使用dMRI对青少年脑震荡发作的儿童进行长期监测是有必要的,重点是脆弱的WM束。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


