Didox, a ribonucleotide reductase inhibitor with iron chelator properties, counteracts the in vitro and in vivo growth of rhabdomyosarcoma cells
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Ribonucleotide reductase (RR) is the rate-limiting enzyme for NTPs conversion into dNTPs, playing a central role in genome replication and maintenance. It is composed by two catalytic (RRM1) and two regulatory (alternatively RRM2 and p53R2) subunits, of which RRM2′s functionality depends on a diferric center in the active site and is one of the most expressed genes in many tumors, among which Rhabdomyosarcoma (RMS), a rare and aggressive pediatric tumor. Didox (3,4-dihydroxy-benzohydroxamic acid) is a highly effective RRM2 inhibitor with iron chelating properties which shows fewer in vivo side effects than classical RR inhibitors. In the present work, we analyzed the impact of didox on RMS cells. Our data clearly showed that didox effectively reduces cell viability, clonogenic capability and motility of both RD and RH30 cells (representative of embryonal named ERMS and alveolar subtype named ARMS), with higher potency in ARMS cells. Interestingly, didox is effective in inhibiting the cell viability of RMS radioresistant. Mechanistically, didox modulates the main iron-related proteins (TfR1 and H-ferritin), confirming its iron chelating properties; it induces mitochondrial ROS formation and caused an increase in double positive annexin-V/PI cells, confirming apoptosis as mechanism of cell death. Moreover, didox also potently reduces in vivo tumor proliferation of RH30 cells, without significant side effects on animals. Finally, the combination of sublethal doses of Actinomycin-D and didox is effective in decreasing cell viability and clonogenicity of RMS cells. Therefore, our data suggests the effectiveness of the RR inhibitor didox on both in vitro and in vivo RMS proliferation.
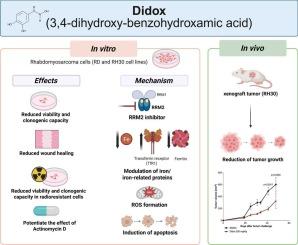
Didox是一种具有铁螯合剂特性的核糖核苷酸还原酶抑制剂,可抑制横纹肌肉瘤细胞的体外和体内生长。
核糖核苷酸还原酶(RR)是NTPs转化为dNTPs的限速酶,在基因组复制和维持中起着核心作用。它由两个催化亚基(RRM1)和两个调节亚基(RRM2和p53R2)组成,其中RRM2的功能依赖于活性位点的差异中心,是许多肿瘤中表达最多的基因之一,其中横纹肌肉瘤(RMS)是一种罕见的侵袭性儿科肿瘤。二dox(3,4-二羟基苯甲羟肟酸)是一种高效的RRM2抑制剂,具有铁螯合特性,与传统的RR抑制剂相比,其体内副作用更小。在本研究中,我们分析了二dox对RMS细胞的影响。我们的数据清楚地表明,二dox有效地降低了RD和RH30细胞(胚胎型称为ERMS和肺泡亚型称为ARMS的代表)的细胞活力、克隆生成能力和活力,在ARMS细胞中具有更高的效力。有趣的是,二dox能有效抑制RMS耐辐射的细胞活力。在机制上,二氧化二钾调节主要的铁相关蛋白(TfR1和h -铁蛋白),确认其铁螯合特性;诱导线粒体ROS形成,双阳性annexin-V/PI细胞增加,证实凋亡是细胞死亡的机制。此外,二dox还能有效降低RH30细胞的体内肿瘤增殖,对动物无明显副作用。最后,亚致死剂量放线菌素- d和二dox联合使用可有效降低RMS细胞的细胞活力和克隆原性。因此,我们的数据表明,RR抑制剂二dox对体外和体内RMS增殖都有效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


