Alpha-lipoic acid attenuates cardiac inflammation of CVB3 induced viral myocarditis via neutrophil-derived YM-1
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-07
DOI:10.1016/j.bbadis.2025.168034
引用次数: 0
Abstract
Background and aims
Viral myocarditis is an inflammatory pathology of the myocardium that involves innate immune responses, especially those involving neutrophils. However, strategies targeting neutrophils to alleviate inflammation have not achieved complete success. Alpha lipoic acid (ALA), a natural organosulfur compound, has the capacity to modulate immune cell behavior. This study aimed to investigate whether ALAs can regulate innate immunity to provide protection against coxsackievirus B3 (CVB3)-induced myocarditis.
Methods and results
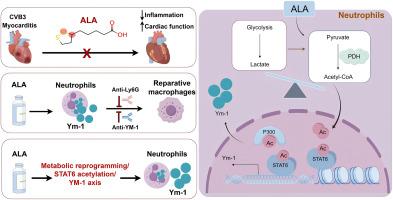
Abdominal administration of ALA improved cardiac dysfunction and reduced mortality in a CVB3-induced mouse model of myocarditis (VM). ALA treatment induced neutrophils and inhibited Ly6Chi pro-inflammatory macrophages, favoring a reparatory environment in the myocardium. However, depleting neutrophils with anti-Ly6G antibodies increased Ly6Chi pro-inflammatory macrophage recruitment in blood and heart in ALA-treated VM mice. Further flow cytometry analysis indicated that ALA promoted Ym-1 expression of neutrophils. Blocking Ym-1 significantly reversed ALA-mediated cardiac reparative macrophages infiltration and cardiac function improvement post-VM. Recombinant Ym-1 drives macrophages toward a reparative phenotype and attenuates CVB3-induced viral myocarditis. Mechanistically, ALA reprogrammed neutrophil metabolic patterns, leading to increased production of the metabolite acetyl-CoA, thereby increasing the transcription of Ym-1 by promoting STAT6 acetylation.
Conclusions
ALA protects against VM by activating metabolic reprogramming/STAT6 acetylation/Ym-1 axis in neutrophils. These results highlight the heterogeneity of neutrophils and suggest that ALA might represent a feasible strategy to improve the prognosis of VM patients.
α -硫辛酸通过中性粒细胞来源的m -1减轻CVB3诱导的病毒性心肌炎的心脏炎症
背景和目的病毒性心肌炎是一种涉及先天性免疫反应的心肌炎症病理,特别是涉及中性粒细胞的免疫反应。然而,针对中性粒细胞减轻炎症的策略尚未取得完全成功。α硫辛酸(ALA)是一种天然有机硫化合物,具有调节免疫细胞行为的能力。本研究旨在探讨ALAs是否可以调节先天免疫,对柯萨奇病毒B3 (CVB3)诱导的心肌炎提供保护。方法和结果腹腔给药ALA可改善cvb3诱导的心肌炎(VM)小鼠心功能障碍,降低死亡率。ALA治疗诱导中性粒细胞,抑制Ly6Chi促炎巨噬细胞,有利于心肌的修复环境。然而,用抗ly6g抗体消耗中性粒细胞会增加ala处理的VM小鼠血液和心脏中Ly6Chi促炎巨噬细胞的募集。进一步流式细胞术分析表明ALA促进了中性粒细胞m-1的表达。阻断m-1可显著逆转ala介导的心脏修复性巨噬细胞浸润和vm后心功能改善。重组m-1驱动巨噬细胞走向修复表型,并减弱cvb3诱导的病毒性心肌炎。从机制上说,ALA重编程中性粒细胞代谢模式,导致代谢物乙酰辅酶a的产生增加,从而通过促进STAT6乙酰化增加m-1的转录。结论sala通过激活中性粒细胞代谢重编程/STAT6乙酰化/ m-1轴对VM有保护作用。这些结果突出了中性粒细胞的异质性,表明ALA可能是改善VM患者预后的可行策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


