SARS-CoV E protein couples asymmetric leaflet thickness and curvature deformations
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
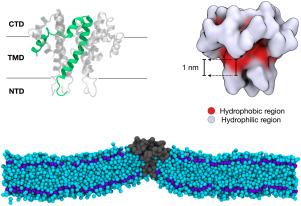
The Envelope protein (E protein) of SARS-CoVs 1 and 2 has been implicated in the viral budding process and maintaining the spherical shape of the virus, but direct evidence linking the protein to long-range membrane deformation is still lacking. Computational predictions from molecular simulation have offered conflicting results, some showing long-range E-induced membrane curvature and others showing only local deformations. In the present study, we determine the mechanism driving these deformations by modulating the degree of hydrophobic mismatch between protein and membrane. We observe that certain barostat and restraint settings, common in coarse-grained MD simulations, can prevent equilibration of the membrane area. Our results indicate that the E protein does not induce long-range curvature, but does exhibit severe local deformations that are exacerbated by hydrophobic mismatch. These deformations occur in conjunction with local leaflet thickness asymmetry, suggesting asymmetry and curvature couple to reduce the free energy cost of a deformed membrane.
SARS-CoV E蛋白偶联不对称小叶厚度和曲率变形
sars - cov 1和2的包膜蛋白(E蛋白)与病毒出芽过程和维持病毒的球形有关,但仍缺乏将该蛋白与远程膜变形联系起来的直接证据。分子模拟的计算预测提供了相互矛盾的结果,一些显示远程e诱导的膜曲率,而另一些只显示局部变形。在目前的研究中,我们通过调节蛋白质和膜之间疏水不匹配的程度来确定驱动这些变形的机制。我们观察到,在粗粒度MD模拟中常见的某些恒压器和约束设置可以阻止膜区域的平衡。我们的研究结果表明,E蛋白不会诱导长程曲率,但确实表现出严重的局部变形,这种变形会因疏水错配而加剧。这些变形与局部小叶厚度不对称一起发生,表明不对称和曲率耦合以减少变形膜的自由能成本。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


