Enantioselective Construction of Fused N-Heterocycles via Sequential Annulation and Catalytic Transfer Hydrogenation
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report the first regio- and enantioselective synthesis of tetrahydropyrido[2,3-b]pyrazines using a chiral iridacycle catalyst. Pyridyl diamines and diketones undergo sequential annulation and asymmetric transfer hydrogenation of the in situ generated pyrido[2,3-b]pyrazine intermediates. This method provides diverse fused N-heterocycles in high yields (up to 95%) and enantioselectivity (98.5:1.5 er), exclusively reducing the pyridyl ring to form the stereocenter at the C6 site. Mechanistic studies reveal that π–π stacking and H-bonding govern the enantioselectivity.

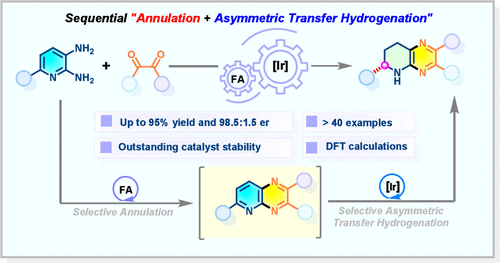
序贯环化和催化转移加氢法制备n -杂环的对映选择性
本文报道了用手性环催化剂首次进行区域选择性和对映选择性合成四氢吡啶[2,3-b]吡嗪。吡啶二胺和二酮经过原位生成的吡啶[2,3-b]吡嗪中间体的顺序环化和不对称转移氢化。该方法提供了多种融合的n -杂环,收率高(高达95%),对映选择性高(98.5:1.5 er),专门还原吡啶环,在C6位点形成立体中心。机理研究表明,π -π堆积和氢键控制了对映体选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


