Cytoprotective effects of Gymnemic Acid 1 in cellular models of neurodegeneration
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Gymnema sylvestre (G. sylvestre) is a traditional medicinal herb known for its anti-diabetic properties, yet its molecular mechanisms remain unknown. Growing evidence suggests a strong link between insulin resistance and neurodegeneration, mediated by impaired pro-survival signaling (e.g., PI3K/Akt), increased oxidative stress, and inflammation.
This study investigates the cytoprotective potential of Gymnemic Acid 1 (GA1), a key bioactive compound from G. sylvestre, in cellular models of neurodegeneration. Neurotoxicity was induced in SH-SY5Y cells using either 6-hydroxydopamine (6-OHDA) or streptozotocin (STZ), followed by treatment with increasing concentrations of GA1. GA1 treatment significantly increased cell viability and reduced malondialdehyde (MDA) levels, indicating antioxidant and cytoprotective effects.
To explore its mechanism of action, we assessed GA1 activity at the glucagon-like peptide-1 receptor (GLP-1R) using a reporter gene assay. GA1 exhibited agonistic activity at GLP-1R, and its protective effects are lost in the presence of a GLP-1R antagonist, further solidifying the involvement of GLP-1R in GA1's action. Further analysis revealed that GA1 treatment increased phosphorylation of PI3K and Akt proteins compared to toxin-only controls, suggesting activation of the GLP-1R/PI3K/Akt signaling pathway.
These findings demonstrate that GA1 confers protection against neurotoxic insults via antioxidant mechanisms and activation of GLP-1R-mediated pro-survival signaling. This study provides mechanistic insight into the neuroprotective potential of G. sylvestre and supports further exploration of GA1 as a candidate for therapeutic development in neurodegenerative diseases.
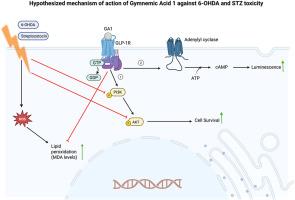
裸子酸1在神经退行性变细胞模型中的细胞保护作用。
Gymnema sylvestre (G. sylvestre)是一种以其抗糖尿病特性而闻名的传统草药,但其分子机制尚不清楚。越来越多的证据表明,胰岛素抵抗与神经退行性变之间存在密切联系,这是由促生存信号(如PI3K/Akt)受损、氧化应激增加和炎症介导的。本研究探讨了金裸子酸1 (GA1)在神经退行性疾病细胞模型中的细胞保护作用。用6-羟基多巴胺(6-OHDA)或链脲佐菌素(STZ)诱导SH-SY5Y细胞神经毒性,然后增加GA1浓度。GA1处理显著提高了细胞活力,降低了丙二醛(MDA)水平,表明其具有抗氧化和细胞保护作用。为了探索其作用机制,我们使用报告基因测定法评估了GA1在胰高血糖素样肽-1受体(GLP-1R)上的活性。GA1对GLP-1R表现出激动作用,在GLP-1R拮抗剂存在时,其保护作用丧失,进一步巩固了GLP-1R参与GA1的作用。进一步分析显示,与仅毒素对照相比,GA1处理增加了PI3K和Akt蛋白的磷酸化,表明激活了GLP-1R/PI3K/Akt信号通路。这些发现表明,GA1通过抗氧化机制和激活glp - 1r介导的促生存信号,对神经毒性损伤具有保护作用。该研究提供了对西尔韦特的神经保护潜力的机制见解,并支持进一步探索GA1作为神经退行性疾病治疗开发的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


