LncRNA HANR promotes the aerobic glycolysis in prostate cancer by stabilizing TPI1
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Prostate cancer (PCa) is a type of malignancy that originates in the prostate gland, often characterized by uncontrolled cell growth and potential metastasis. Long non-coding RNAs (lncRNAs) play crucial regulatory roles in the progression of prostate cancer, potentially facilitating tumor growth and metastasis via mechanisms that involve the enhancement of aerobic glycolysis. This study aimed to investigate the functional role of lncRNA HANR in prostate cancer progression. Bioinformatics analysis and experimental validation revealed a significant up-regalation of HANR in prostate cancer tissues, in comparison to adjacent normal tissues. Functional studies demonstrated that silencing HANR inhibited prostate cancer cells proliferation, migration, invasion, and glycolysis. While HANR overexpression promoted prostate cancer cells proliferation, invasion, and glycolysis. Mechanistically, HANR interacts with triosephosphate isomerase 1 (TPI1), a key glycolytic enzyme, to promote glycolysis and tumor growth. Silencing HANR or TPI1 reduced prostate tumor growth both in vitro and in vivo. In conclusion, our findings suggest that the HANR-TPI1 axis plays a crucial role in the progression of prostate cancer and may represent a novel biomarker and therapeutic target for aggressive prostate cancer, given its role in enhancing aerobic glycolysis and facilitating tumorigenesis in prostate cancer cells.
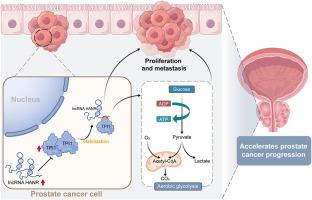
LncRNA HANR通过稳定TPI1促进前列腺癌的有氧糖酵解。
前列腺癌(PCa)是一种起源于前列腺的恶性肿瘤,通常以细胞生长不受控制和潜在的转移为特征。长链非编码rna (lncRNAs)在前列腺癌的进展中起着至关重要的调节作用,可能通过涉及增强有氧糖酵解的机制促进肿瘤的生长和转移。本研究旨在探讨lncRNA HANR在前列腺癌进展中的功能作用。生物信息学分析和实验验证显示,与邻近正常组织相比,前列腺癌组织中HANR显著上调。功能研究表明,沉默HANR可抑制前列腺癌细胞的增殖、迁移、侵袭和糖酵解。而HANR过表达促进前列腺癌细胞的增殖、侵袭和糖酵解。从机制上讲,HANR与糖酵解关键酶三磷酸异构酶1 (TPI1)相互作用,促进糖酵解和肿瘤生长。在体外和体内,沉默HANR或TPI1均可减少前列腺肿瘤的生长。总之,我们的研究结果表明,HANR-TPI1轴在前列腺癌的进展中起着至关重要的作用,并且可能代表一种新的生物标志物和侵袭性前列腺癌的治疗靶点,因为它可以增强前列腺癌细胞的有氧糖酵解和促进肿瘤发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


