Ras/Raf dimerization model for activation of Raf kinase
IF 6.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Our previously proposed Ras dimerization model is consistent with recent details observed by NMR in that Raf activation is centered on the Ras/Raf dimer, distinct from one in which Ras activates Raf as a monomer with the Raf cysteine rich domain inserted in the membrane. We review mechanistic understanding of Raf activation within nanoclusters of Ras on the membrane, with a shift to dimers upon binding Raf. This sets the stage for a signaling platform composed of Ras/Raf and Galectin dimers that facilitates the release of Raf autoinhibition and folding of the Raf intrinsically disordered region between the Ras-binding domains and the kinase bound to 14-3-3 and MEK. This platform could provide synchronized units for signal amplification and is consistent with a Ras stationary phase observed in cells.
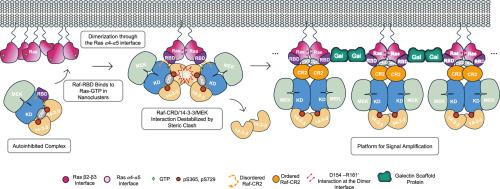
激活Raf激酶的Ras/Raf二聚化模型
我们之前提出的Ras二聚化模型与最近通过核磁共振观察到的细节一致,因为Raf的激活集中在Ras/Raf二聚体上,与Ras激活Raf作为一个单体并在膜中插入Raf半胱氨酸富结构域的模型不同。我们回顾了膜上Ras纳米簇内Raf激活的机制,并在结合Raf时转向二聚体。这为一个由Ras/Raf和半乳糖凝集素二聚体组成的信号传导平台奠定了基础,该平台促进了Raf自抑制的释放和Ras结合域与14-3-3和MEK结合的激酶之间Raf内在无序区域的折叠。该平台可以为信号放大提供同步单元,并且与细胞中观察到的Ras固定相一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current opinion in structural biology
生物-生化与分子生物学
CiteScore
12.20
自引率
2.90%
发文量
179
审稿时长
6-12 weeks
期刊介绍:
Current Opinion in Structural Biology (COSB) aims to stimulate scientifically grounded, interdisciplinary, multi-scale debate and exchange of ideas. It contains polished, concise and timely reviews and opinions, with particular emphasis on those articles published in the past two years. In addition to describing recent trends, the authors are encouraged to give their subjective opinion of the topics discussed.
In COSB, we help the reader by providing in a systematic manner:
1. The views of experts on current advances in their field in a clear and readable form.
2. Evaluations of the most interesting papers, annotated by experts, from the great wealth of original publications.
[...]
The subject of Structural Biology is divided into twelve themed sections, each of which is reviewed once a year. Each issue contains two sections, and the amount of space devoted to each section is related to its importance.
-Folding and Binding-
Nucleic acids and their protein complexes-
Macromolecular Machines-
Theory and Simulation-
Sequences and Topology-
New constructs and expression of proteins-
Membranes-
Engineering and Design-
Carbohydrate-protein interactions and glycosylation-
Biophysical and molecular biological methods-
Multi-protein assemblies in signalling-
Catalysis and Regulation
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


